Good Buffer Examples
For example, when a buffer annuity offers a 10% buffer against losses, the insurance company offering the product will absorb the first 10% of losses associated with the product.

Good buffer examples. The most important physiological buffers in the body are the bicarbonate–CO 2 system, the large anion complexes such as plasma proteins and phosphates and hemoglobin in cells. It depends, most DAWs will have different buffer size 32, 64, 128, 256, 512 and 1024, when you are recording, you need to monitor your input signal in real time, so choosing lower buffer size like 32 or 64 with quicker information processing speed to avoid latency. Set the buffer size to a lower amount to reduce the amount of latency for more accurate monitoring.
A buffer solution was made by dissolving 10.0 grams of sodium acetate in 0.0 mL of 1.00 M acetic acid. Historically, one can look to examples such as Afghanistan — a buffer between British India and Czarist Russia — or Belgium — a buffer between France, the German Empire, and the Netherlands in the decades before World War I. If the buffer size is set too high while recording, however, there will be quite a bit of latency which can be frustrating.
The LibreTexts libraries are Powered by MindTouch ® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. For example, the following could function as buffers when together in solution:. This is how we do it at Buffer!.
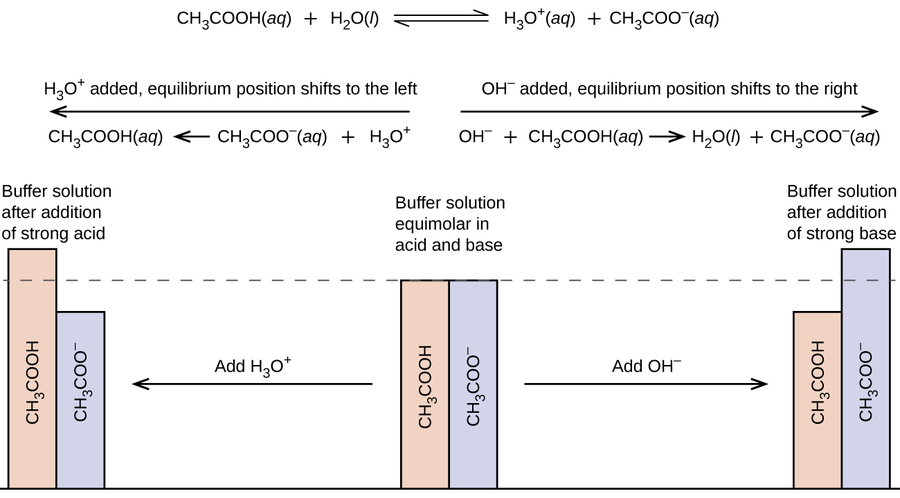
An example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (pH = 4.75). If acid is added to this buffer, the added H + ions combine with bicarbonate ions to produce more carbonic acid, using up some of the H + ions (the Na + ions do not. An alkaline buffer solution has a pH greater than 7.
As described in How Buffer works, an important feature of the Buffer tool is the Method parameter which determines how buffers are constructed. If you add an acid or a base to a buffered solution, its pH will not change significantly. Learn more about how Buffer works.
It explains the concept, compon. The buffer size is the amount of time you allocate to your DAW for processing audio. Buffer capacity is a property of a buffer and it tells you how much acid or base you can add before the pH starts changing.
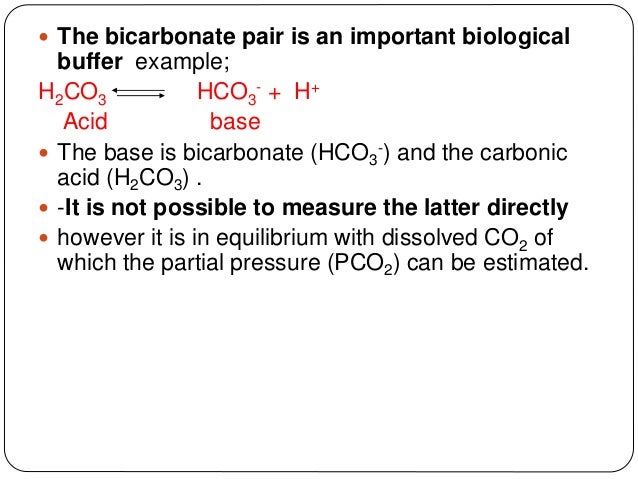
Some real-life 5 Whys examples. A buffer of carbonic acid (H2CO3) and hydrogen carbonate (HCO3-), for example, work in unison to keep the pH of the bloodstream at a neutral 7.4. Most biological reactions take place at a pH between 6 and 8, so ideal buffers have pKa values in this range to provide maximum buffering capacity there.
A higher sample rate can also capture ultrasonic frequencies. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. They can be further delineated by grade.
In all of these, the essential reaction is:. And finally – buffer size. In this system, the weak acid dissociates to a small extent, giving bicarbonate ions.
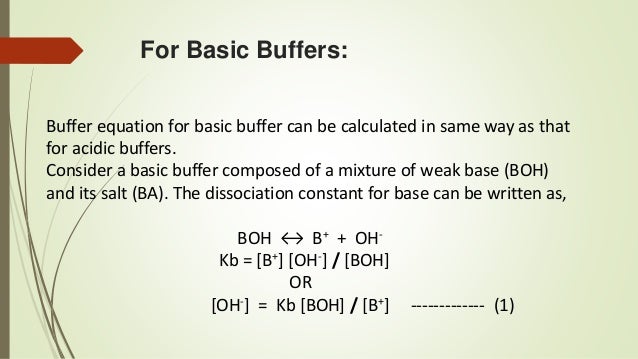
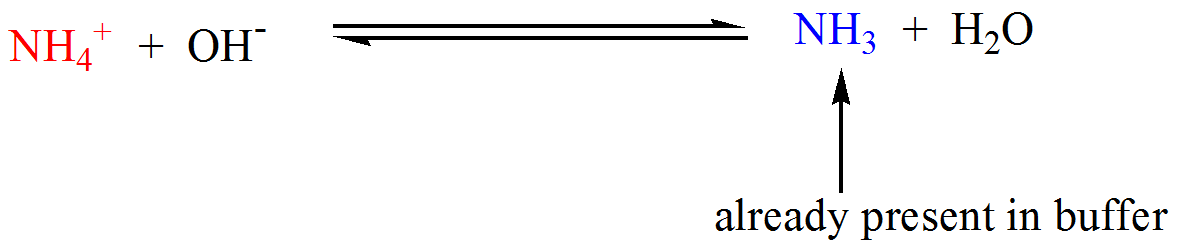
Alkaline buffers, on the other hand, have a pH above 7 and contain a weak base and one of its salts. A common weak base buffer is made of ammonia (NH3) and ammonium chloride (NH4Cl). It has good pH buffering capacity within the range of pH 5.0–7.4.
K b for ammonia equals 1.77 x 10-5. Tris is a mid-range buffer stable at pH of 7 to 9.6. In early 14, we had a brief systemwide outage.
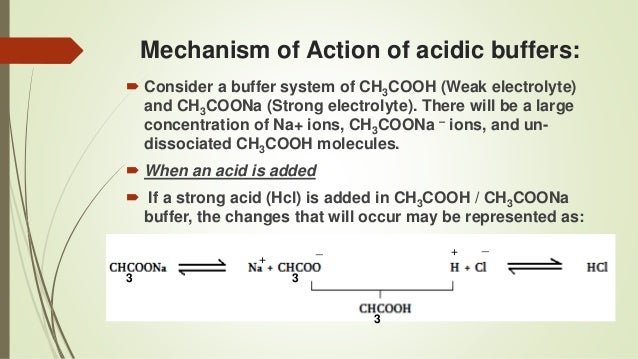
The table above is an example of a look-up table. Cacodylate was introduced for electron microscopy applications by Sabatini et al. Acetic acid (weak organic acid w/ formula CH 3 COOH) and a salt containing its conjugate base, the acetate anion (CH 3 COO - ), such as sodium acetate (CH 3 COONa).
For example, blood in the human body is a buffer solution. Alkaline buffer solutions are commonly made from a weak base and one of its salts. To take the 5 Whys from theoretical to actual, here’s a look at a few moments in Buffer’s history that have called for a 5 Whys meeting.
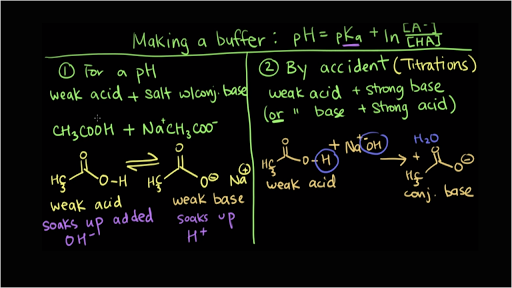
Buffer solutions are those which retain their pH when a small amount of acid or base is added. There are two main situations where you make a buffer, and for lack of a better way to put it, the first way is when you know you want a buffer with a specific pH, so you're making it on purpose for a specific application, and then the second time that you might make a buffer, especially in chemistry class, is. Here’s a look at the 5 Whys the team conducted:.
Use the table from the label that is specific to the application method. Most buffers work best at a pH within 1. Most of the buffers were new zwitterionic compounds prepared and tested by Good and coworkers for the first time, though some were known compounds previously overlooked by biologists.
Using the example table above, if an applicator applies metam sodium by shank injection to beds at the broadcast equivalent rate of 75 gallons per acre (gal/acre) to an application block that is 1 acres, the buffer zone is 129 feet. Connectivity of buffers is essential. A buffer is a mixture of an acid that does not ionize completely in water and its corresponding base-for example, carbonic acid (H 2 CO 3) and sodium bicarbonate (NaHCO 3).
The following code example illustrates the use of several Buffer class methods. This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. If you have a Tris buffer prepared at °C with a pK a of 8.3, it would be an effective buffer for many biochemical reactions (pH 7.3–9.3), but the same Tris buffer used at 4°C becomes a poor buffer at pH 7.3 because its pK a shifts to 8.8.
Some are acidic while others are basic. For example, if you must notify someone of a refusal of his loan request, you could open your letter with a pleasantry, such as “I appreciate your time and patience during this loan evaluation process.”. INTERIOR FOREST BUFFER CONNECTIVITY OF BUFFERS RIPARIAN BUFFER.
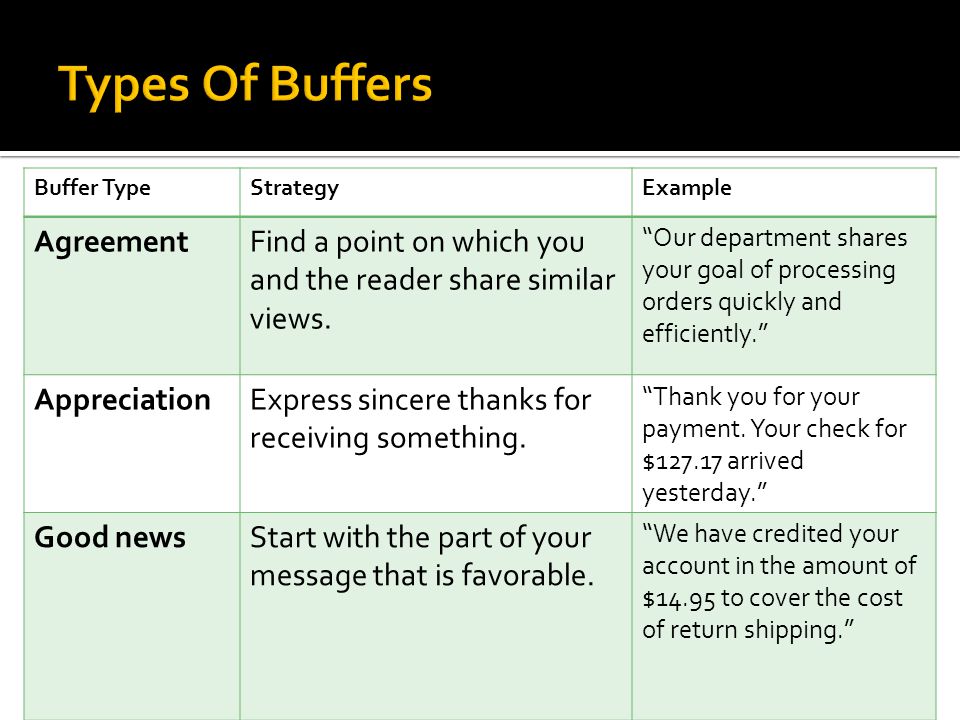
There are two basic methods for constructing buffers:. Your buffer should relay some positive aspect of the negative news you wish to impart. Buffer Reason Bad News Closing.
(1) provide a buffer to cushion the bad news that will follow, (2) let the receiver know what the message is about without stating the obvious, and (3) serve as a transition into the discussion of reasons without revealing the bad news or leading the receiver to expect good news. A buffer state is typically a smaller, neutral state situated between two greater states or political entities. There is no ‘good’ or ‘bad’ setting for buffer size.
For ( int loopX = arr. Additives that act as buffers usually comprise metal salts corresponding to a weak acid found naturally within the food to be preserved. // Example of the Buffer class methods.
Interior forest and upland buffers can protect interior forest areas, provide connectivity between different habitat types, and reduce disturbances to critical breeding and nesting areas. For example, if you prepare a Tris buffer at pH 7.0 in the cold room at 4.0°C, and perform a reaction in that same buffer at 37°C, the pH will drop to 5.95. (1962) as a method of avoiding adding additional phosphates to sample preparations.
Basically, as your buffer capacity goes up, which I'm going to abbreviate BC, as your buffer capacity goes up, you can add more of your acid or base before the pH starts changing a lot. An example of a weak basic solution is seawater, which has a pH near 8.0, close enough to neutral that well-adapted marine organisms thrive in this alkaline environment. 1) This is a buffer solution, with a weak base (the ammonia) and the salt of the weak base (the ammonium chloride) in solution at the same time.
Void DisplayArray( array<short>^arr ) { Console::Write( " arr:" );. PKa value between 6.0 and 8.0, high solubility, non toxic, limited effect on biochemical reactions, very low absorbence between 240 nm and 700 nm, enzymatic and hydrolytic stability, minimal changes due to temperature and concentration, limited effects due to ionic or salt composition of the solution, limited interaction with mineral cations, and limited permeability of biological membranes. For example, a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 4.75.
An example of a common buffer is a solution of acetic acid (CH3COOH) and sodium. At the acidic end of the pH scale, at a range from 5.5 to 8.3 are the morpholinic buffers such as MES, MOPS, and MOBS. Introductory Paragraph "The introductory paragraph in the bad-news message should accomplish the following objectives:.
Similarly, adding water to a buffer or. Ions are atoms or molecules that have lost or gained one or more electrons. A frequently used example is a mixture of ammonia solution and ammonium chloride solution.
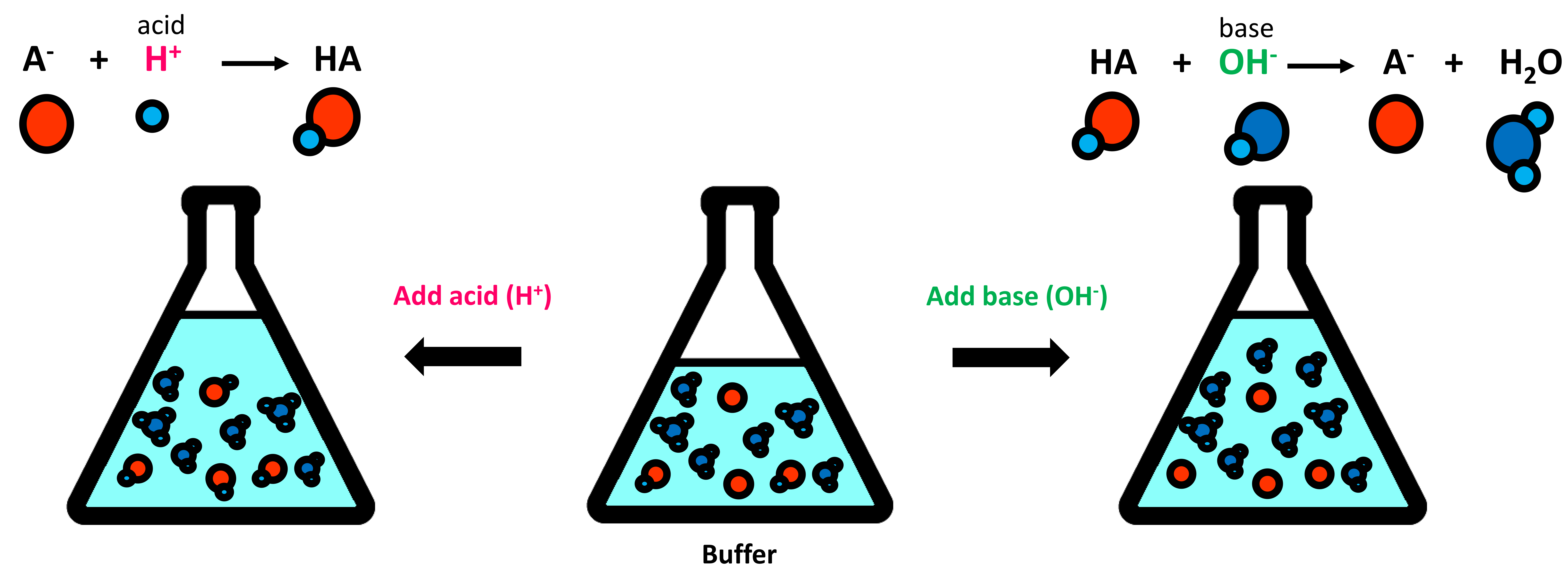
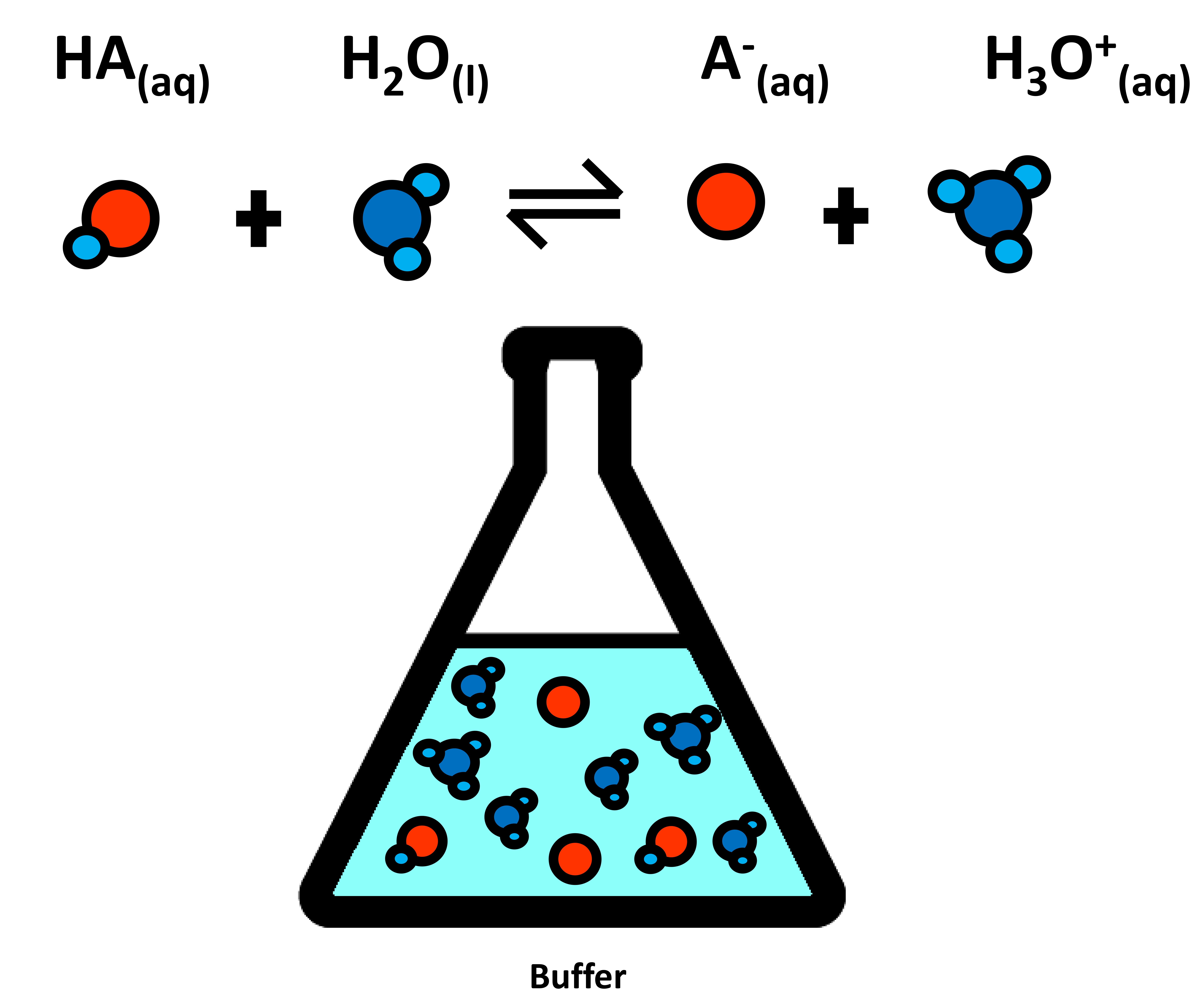
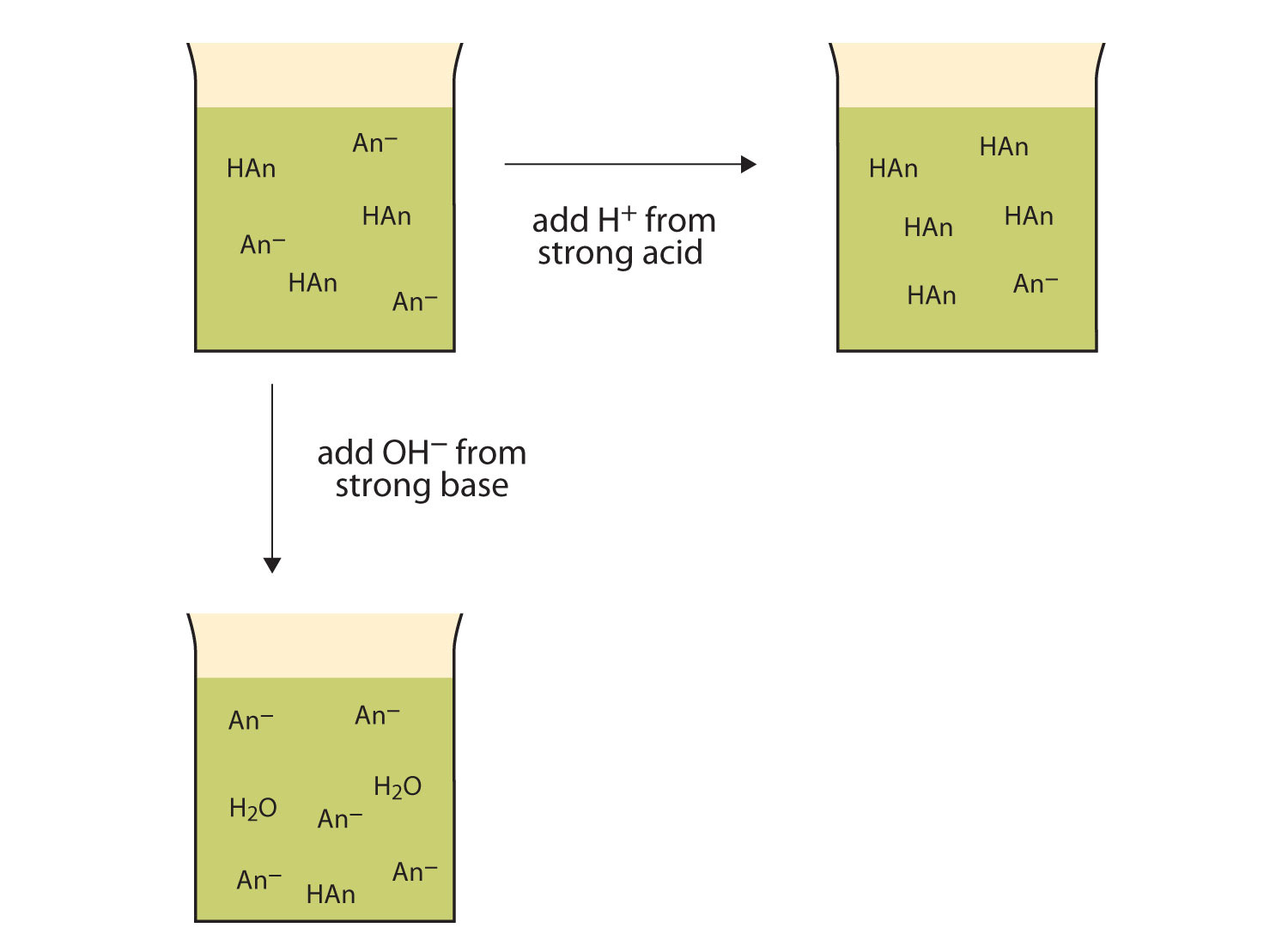
Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. There a number of criteria to consider for choosing the right buffer for an experiment. Buffer solutions are resistant to pH change because of the presence of an equilibrium between the acid (HA) and its conjugate base (A –).
And the corrective actions that resulted:. 4/12/09 2:12:03 PM. The buffer statement should always remain neutral, and never give the reader the impression the succeeding message will contain good news, when that clearly isn't the case.
For example, blood contains a carbonic acid (H 2 CO 3)-bicarbonate (HCO 3-) buffer system. By the way, we’re talking about live performance here, not recording which should be done slightly differently. We also acknowledge previous National Science Foundation support under grant numbers , , and.
Another example of buffers within the human body is the "hemoglobin" complex, which binds to excess protons (in other words, hydrogen ions) muscles release during exercise so that the body can use the. Acid Buffers are a mixture of a weak acid and it's salt Example:. A classic example of a weak acid based buffer is acetic acid (CH3COOH) and sodium acetate (CH3COONa).
Cyclohexylamino buffers such as CHES and CAPS are effective at higher pH values from 8.6 to 11.4. // Display the array elements from right to left in hexadecimal. For example, the addition of sodium citrate to a food.
A buffer is an aqueous solution that has a highly stable pH.A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base. Second, this memo lacks you-attitude and is written from the writer's own viewpoint. Good's buffers are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues during 1966–1980.
This reforms the weak acid and reduces the amount of H + ions in solution. Example of a species that could be. Buffering agents have variable properties—some are more soluble than others;.
EXAMPLES OF BAD NEWS MEMOS. Before Good's work, few hydrogen ion buffers between pH 6 and 8 had been accessible to biologists, and very inappropriate, toxic, reactive and inefficient. - Voiceover In this video we're going to be talking about how you make a buffer.
That is, the function of a buffering agent is to prevent a rapid change in pH when acids or bases are added to the solution. The K a for acetic acid is 1.7 x 10 -5. Some people argue that the lack of these frequencies interferes with your audio.
Rather than have a full diagram of where everyone sits in a hierarchical structure, we’ve leaned into the foundational elements of an org chart — clarity, order, and communication — and extrapolated those bits into a table format. Here are a few tips when working with the buffer size. Creates buffer polygons around input features to a specified distance.
The buffer statement will help bring your reader into a positive frame of mind, before delivering the bad news, but it's a very careful balance between buffering and misleading. If hydrogen ion increases, then it combines with the buffer, if it decreases, some hydrogen ions are released from the. An aqueous solution of an equal concentration of acetic acid and sodium acetate has a pH of 4.74.
If I cut my sample buffer in half to 128 samples – I would save about 3ms of latency or approximately 3ft of distance between me and my monitor. H + + buffer ⇔ H-buffer. Sodium cacodylate buffer Na(CH3)2 AsO2 • 3H2O is a alternative to Sørensen’s phosphate buffer.
Here are the main selection factors:. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. 44.1, 48, .2, 96, 176.4, 192 kHz.
These solutions consist of a weak acid and a salt of a weak acid. For example, a mixture of ammonium chloride and ammonium hydroxide acts as a buffer solution with a pH of about 9.25. How can organisms whose bodies require a near-neutral pH ingest acidic and basic substances (a human drinking orange juice, for example) and survive?.
The characteristics associated with a Good′s buffer include the following:. What Is a Buffer?. To prepare the reader and to try to get the reader to understand the reasoning, the writer should place a buffer and the reasons before the news.
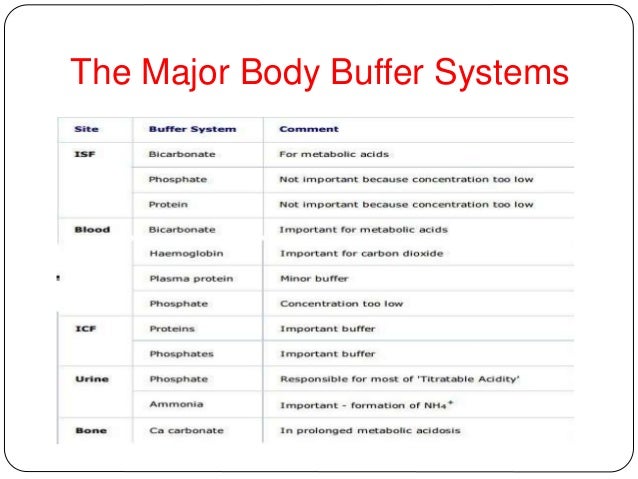
Small molecules such as bicarbonate and phosphate provide buffering capacity as do other substances, such as hemoglobin and other proteins. 0 By using organic products, you help create a buffer between your family and dangerous chemicals that might later be banned, but only after they've been marketed, used and made people sick. Sample Rate values are typically written in kHz (kilohertz).
If these were mixed in equal molar proportions, the solution would have a pH of 9.25. This is why I personally use a buffer size of 256 and sample rate of 44.1KHz. Determine the pH of a solution prepared by dissolving 0.35 mole of ammonium chloride in 1.0 L of 0.25 M aqueous ammonia.
An example of a customer-centric org chart 7. Assuming the change in volume when the sodium acetate is not significant, estimate the pH of the acetic acid/sodium acetate buffer solution. These ions are capable of binding extra H + ions floating around in the blood.
As an example, when recording using a sample rate of 48kHz, (forty-eight thousand) samples are being captured each second by your audio recording. Buffers are the key. PH of these solutions is below seven.
An organic farm, for example, will need a large enough buffer zone to prevent contamination by chemicals sprayed on the crops of non-organic neighboring farms. The downside to lowering the buffer size is that it. Sodium acetate + Acetic acid Borax + Boric acid Basic Buffers are a mixture of a weak b.
The writer does a good job of using a common ground statement and. The Sample Rate is the number of audio samples that are captured per second.

Buffer System In Chemistry Definition Overview Video Lesson Transcript Study Com

Chem 4551 Titration Ph And Buffers Name Date 1 Chegg Com
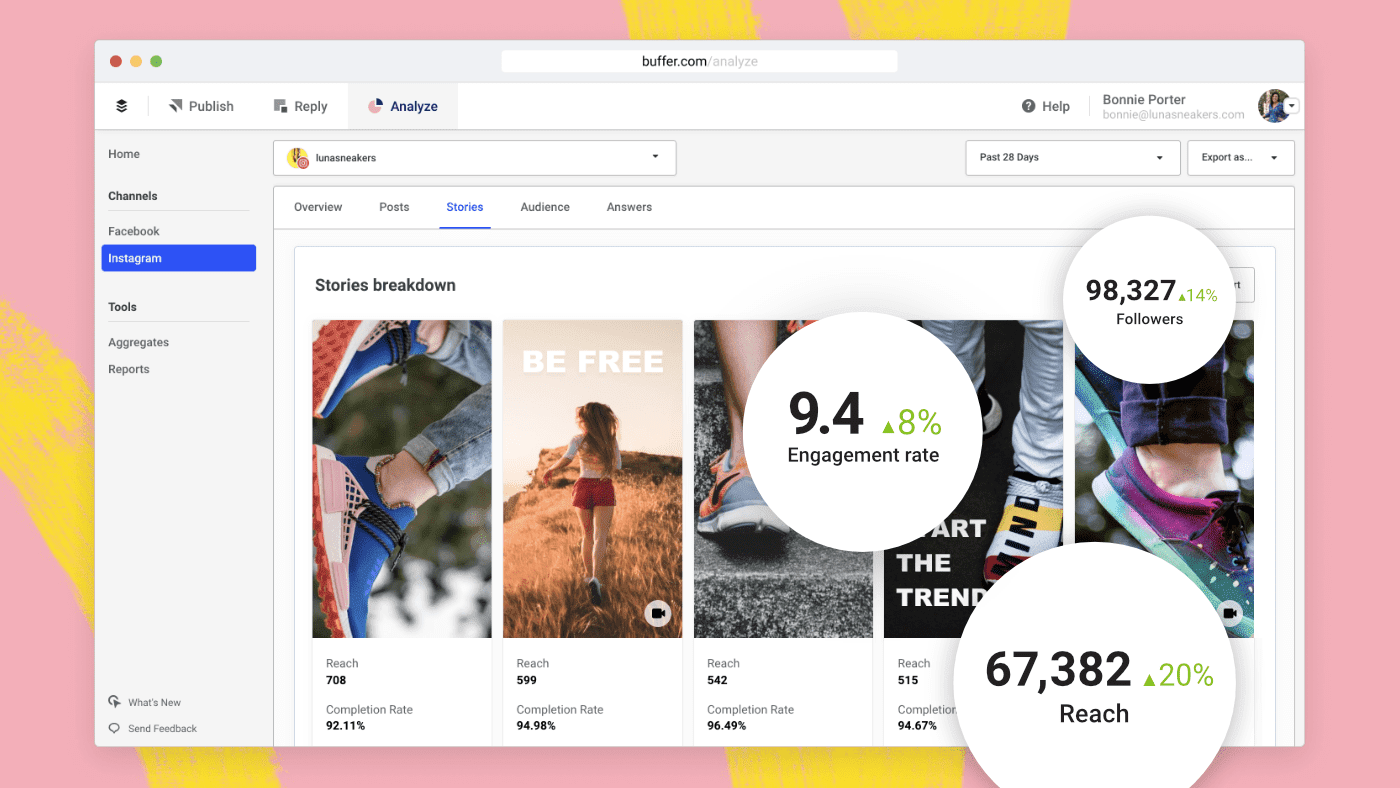
Social Media Analytics Grow Your Following With Insights
Good Buffer Examples のギャラリー
Ways To Get A Buffer Solution Video Khan Academy

What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
Buffers In The Body
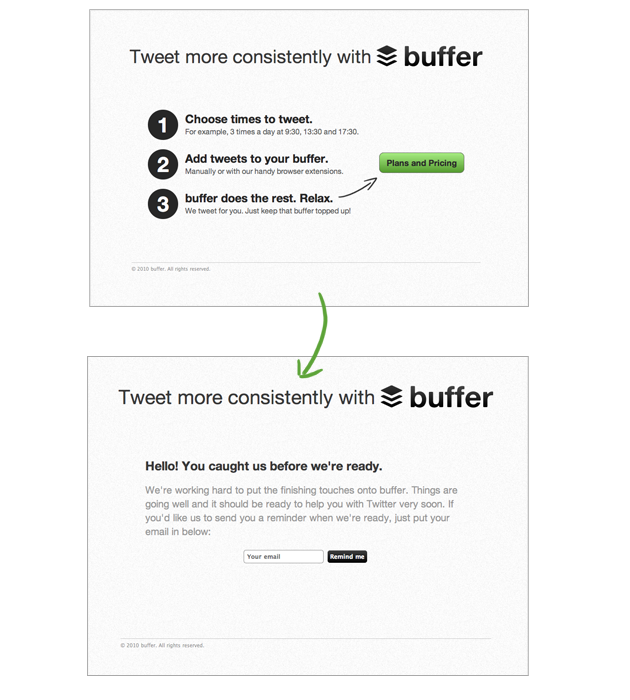
Idea To Paying Customers In 7 Weeks How We Did It

Buffer Solution Wikipedia

How To Write About An Unpleasant News

What Is A Buffer In Chemistry Example

Company Examples For Chapter 8 Bad News Messages Bizcom In The News

Ppt Goals In Communicating Bad News Example Request For Donation From Your Company Powerpoint Presentation Id
Buffers

Buffer Stocks Economics Help

How To Write About An Unpleasant News

Buffer System In Chemistry Definition Overview Video Lesson Transcript Study Com

How To Write About An Unpleasant News

Buffers
7 1 Acid Base Buffers Chemistry Libretexts

The 10 Buffer Values And How We Act On Them Every Day
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
How To Send Better Email Try These Ready To Use Templates Today
Writing Negative News Messages Ppt Download
Promotional Emails 33 Examples Ideas Best Practices Updated

Buffer System In Chemistry Definition Overview Video Lesson Transcript Study Com

Buffering Agent Wikipedia

Buffer Reference Center Sigma Aldrich
2

Chapter 11 Bad News Messages Ppt Download

10 Key Points For Delivering Bad News
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio

Examples Of Good And Bad Buffer Subtractions Download Scientific Diagram

3 Examples Of Buffer Systems Fullexamscom Induced Info
Q Tbn And9gctcbuqe03l5k5hvzcdic4gv4ke9rd5na4somncv8rpq5ak6i25x Usqp Cau
21 B2b Email Marketing Examples Incl Unique Templates
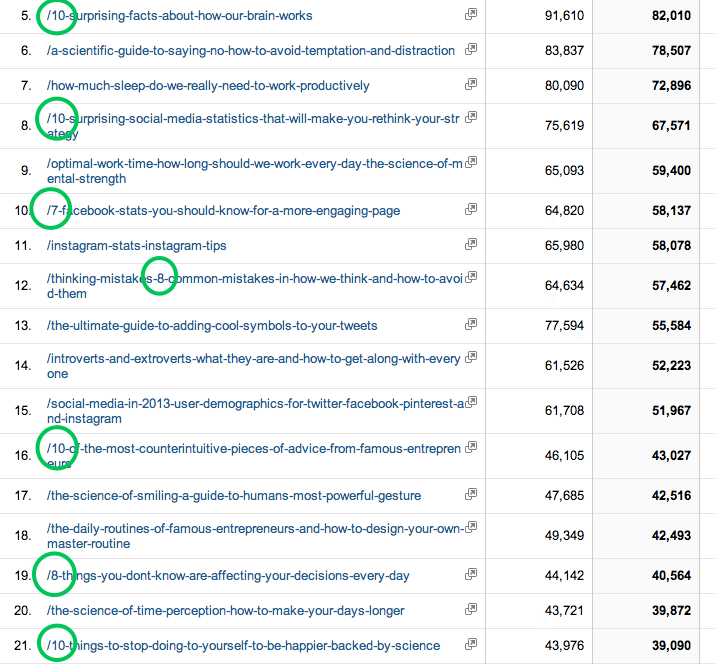
The Marketer S Guide To Google Analytics How To Extract Numbers That Drive Action

Writing Bad News Messages Ppt Video Online Download
Q Tbn And9gctcbdau4xdo7ed63iynmajzvcz0ig3x0oqt5eujwtjbuytmu4wy Usqp Cau

Buffer Solutions Definition Types Preparation Examples And Videos
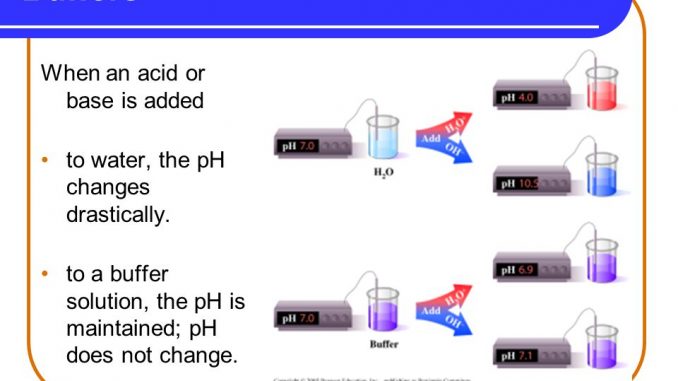
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
Examples Of Good And Bad Buffer Subtractions Download Scientific Diagram
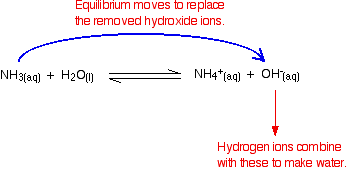
Buffer Solutions
Http Print Ispub Com Api 0 Ispub Article 4408

Buffers
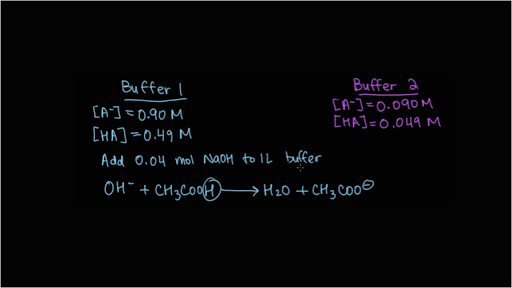
Buffer Capacity Video Buffer Solutions Khan Academy
How To Build Company Culture Our 5 Biggest Learnings

Introduction To Buffers Chemistry Libretexts
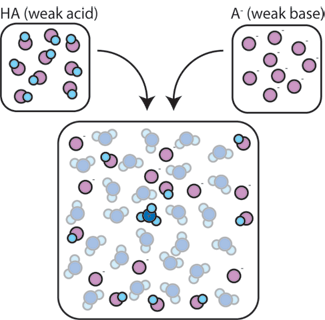
How Buffers Work
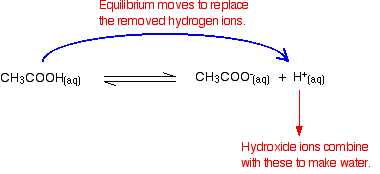
Buffer Solutions

The 10 Buffer Values And How We Act On Them Every Day
Q Tbn And9gcrmtrck5xh9w4oxdodhyowvjh B9a5z0xes54ilnp8nyvwyptdt Usqp Cau

Maintaining Cellular Conditions Ph And Buffers
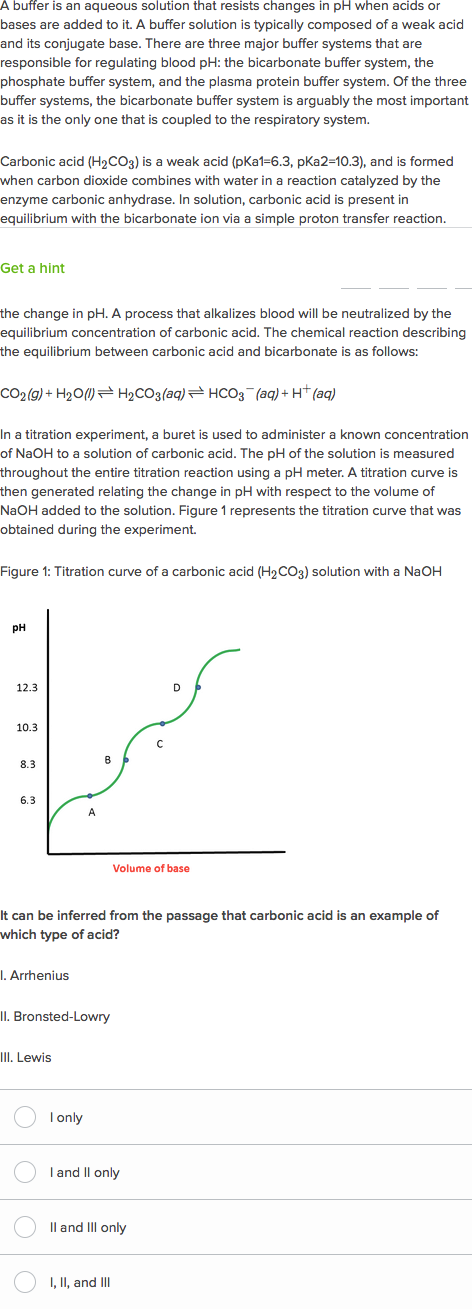
The Role Of The Bicarbonate Buffer System In Regulating Blood Ph Practice Khan Academy

How To Choose The Perfect Buffer To Get A Pure Stabilised Functional Protein Tebu Bio S Blog

Buffer Solutions Definition Types Preparation Examples And Videos

Good News And Bad News
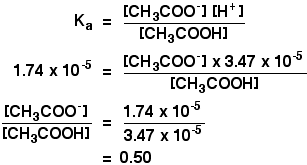
Buffer Solutions

Buffer Zone Wikipedia
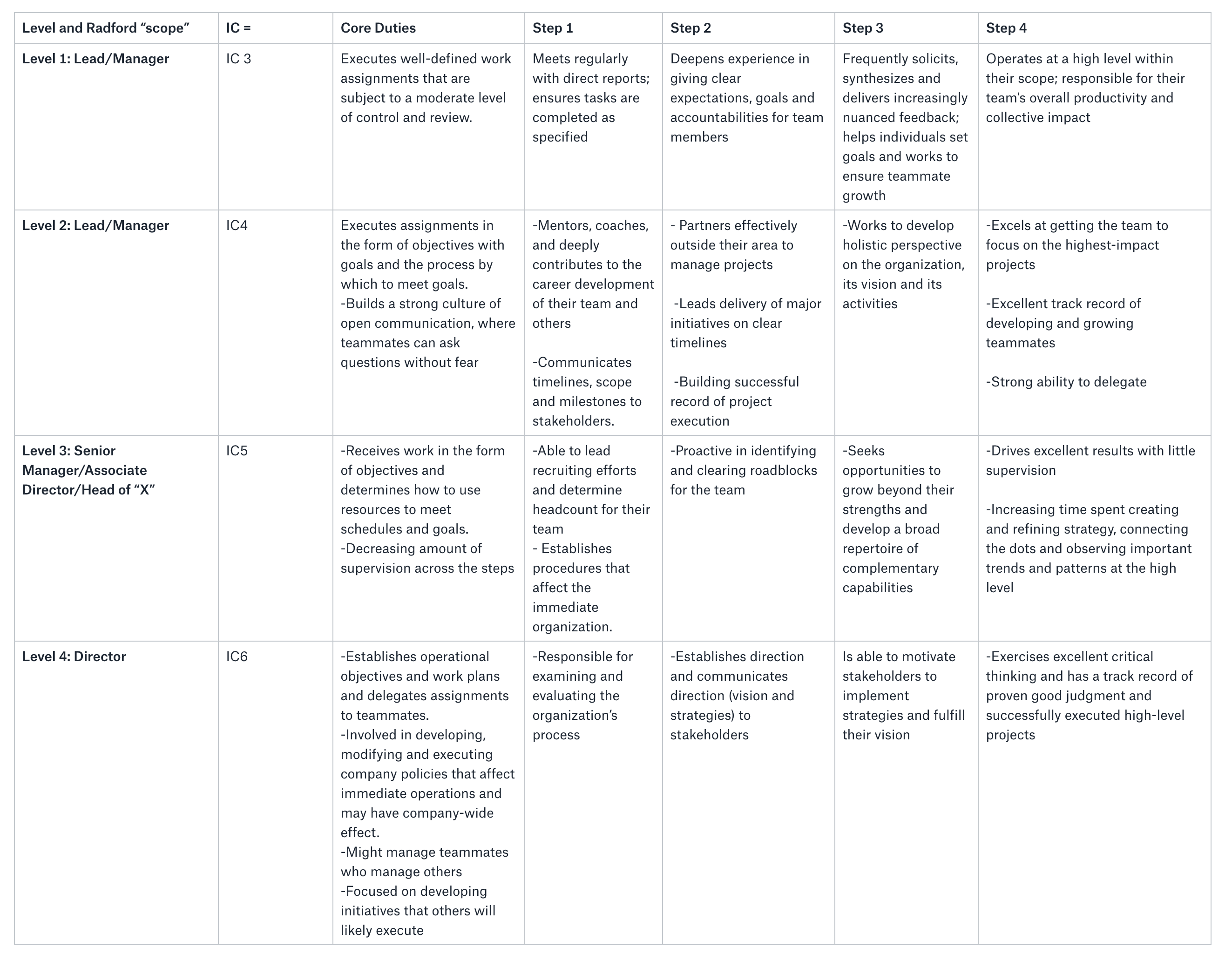
How Individuals Advance At Buffer Without Becoming Managers

Buffer Solutions Definition Types Preparation Examples And Videos
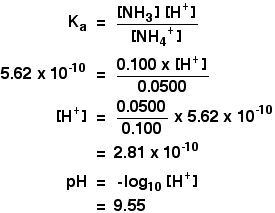
Buffer Solutions

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Buffers

Representative Examples Of The Excellent And Good Classes Of Reases Download Scientific Diagram

Buffer System In Chemistry Definition Overview Video Lesson Transcript Study Com

Buffers

Solved Chem 1152l Lab Manual Page 50 Pre Lab For Ph Of A Chegg Com
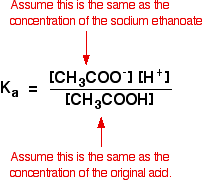
Buffer Solutions

Tips For Iding If A Solution Is A Buffer Concept Chemistry Video By Brightstorm

Good News And Neutral Messages
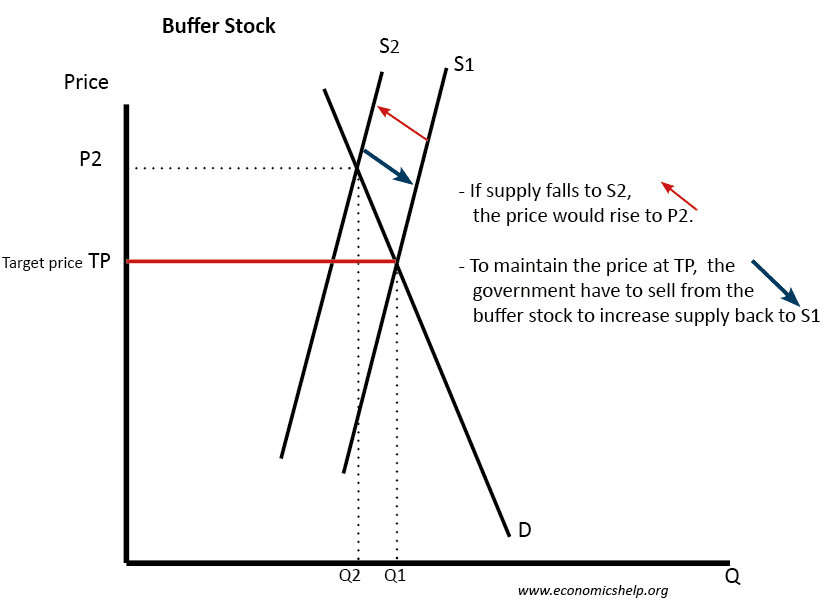
Buffer Stocks Economics Help
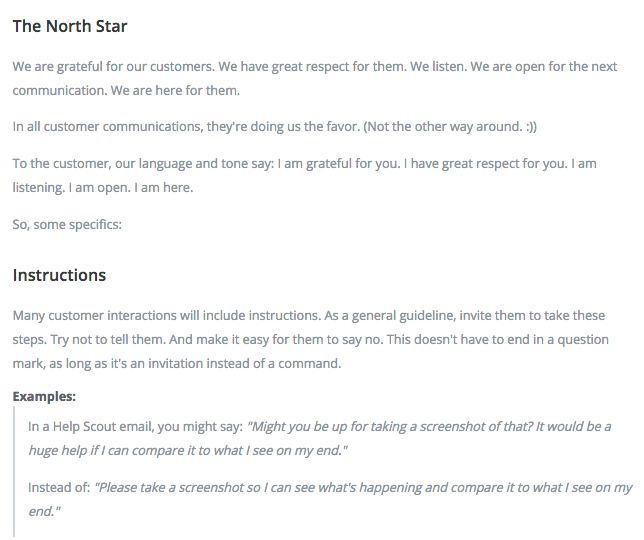
The 10 Buffer Values And How We Act On Them Every Day

How To Choose The Perfect Buffer To Get A Pure Stabilised Functional Protein Tebu Bio S Blog
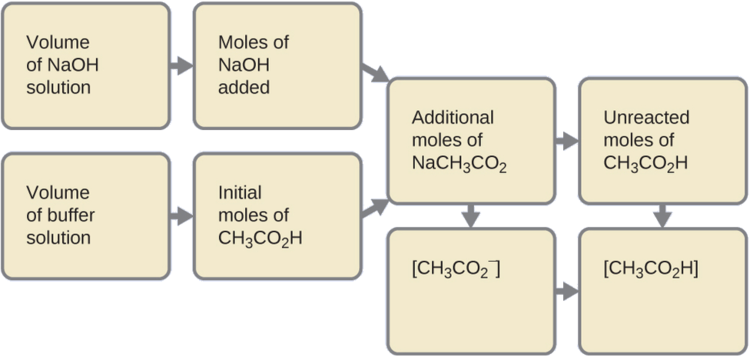
14 6 Buffers Chemistry Libretexts

Ph And Pka Relationship For Buffers Video Khan Academy
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
Company Examples For Chapter 8 Bad News Messages Bizcom In The News

Solved 2 What Are The Requirements Of A Good Buffer Sho Chegg Com

How To Write About An Unpleasant News

Maintaining Cellular Conditions Ph And Buffers

Writing Bad News Messages Ppt Video Online Download

Solved 2 Some Students Are Surprised That The Ph Of Dist Chegg Com

Ways To Get A Buffer Solution Video Khan Academy
Buffers

Pdf Comparison Of Zwitterionic N Alkylaminomethanesulfonic Acids To Related Compounds In The Good Buffer Series Semantic Scholar

Ph Buffers In The Blood

Buffers Pharmaceutics

Buffer Solution Ph Calculations Video Khan Academy

Buffer Solutions Definition Types Preparation Examples And Videos

Biological Buffers Sigma Aldrich

Buffer Mayerseidman Ah Good One Mayer Have Any Examples Of Others Doing This Well Mary
8 Powerful Features To Help You Use Buffer To The Fullest
Buffers In The Body
Company Examples For Chapter 8 Bad News Messages Bizcom In The News

Solved 5 Co2 Is Soluble In Water Based On This Explain Chegg Com
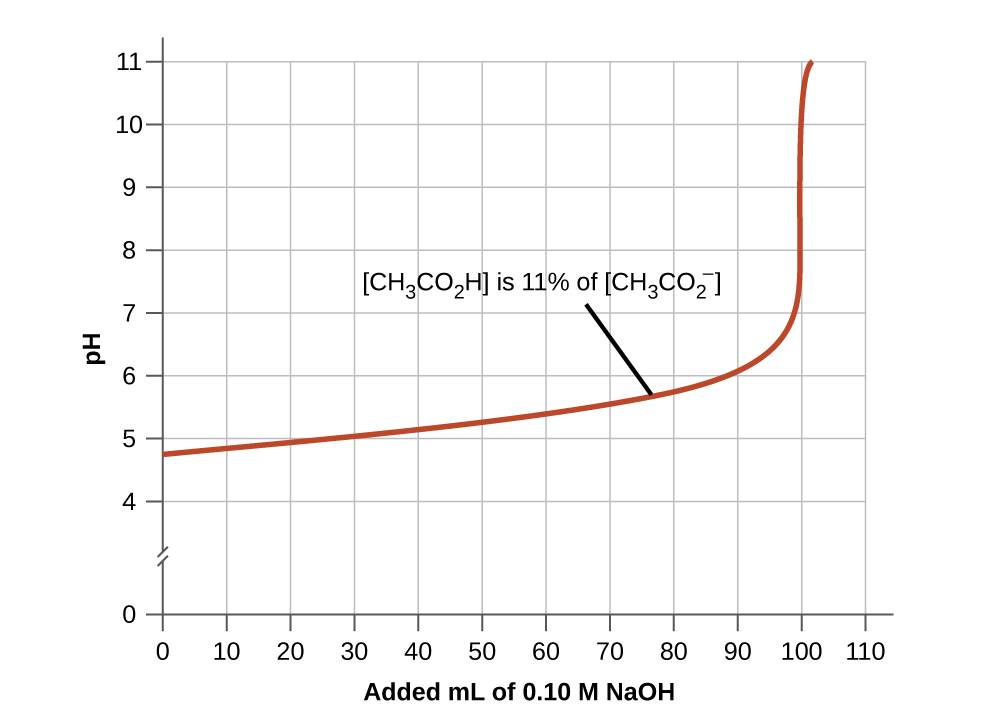
14 6 Buffers Chemistry Libretexts
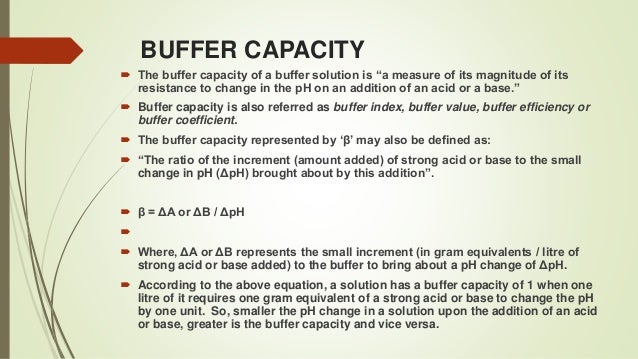
Buffers
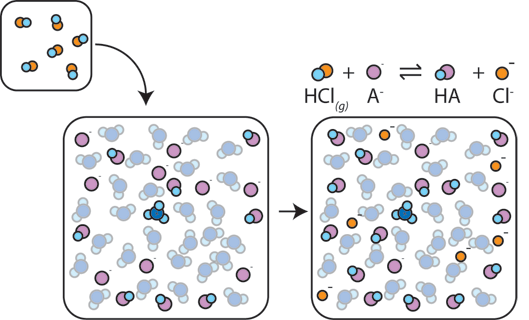
How Buffers Work

Solved 5 Give Examples Of How Ph May Affect Biological R Chegg Com

What Makes A Good Buffer Promega Connections

Characteristics Of Good Buffers
1
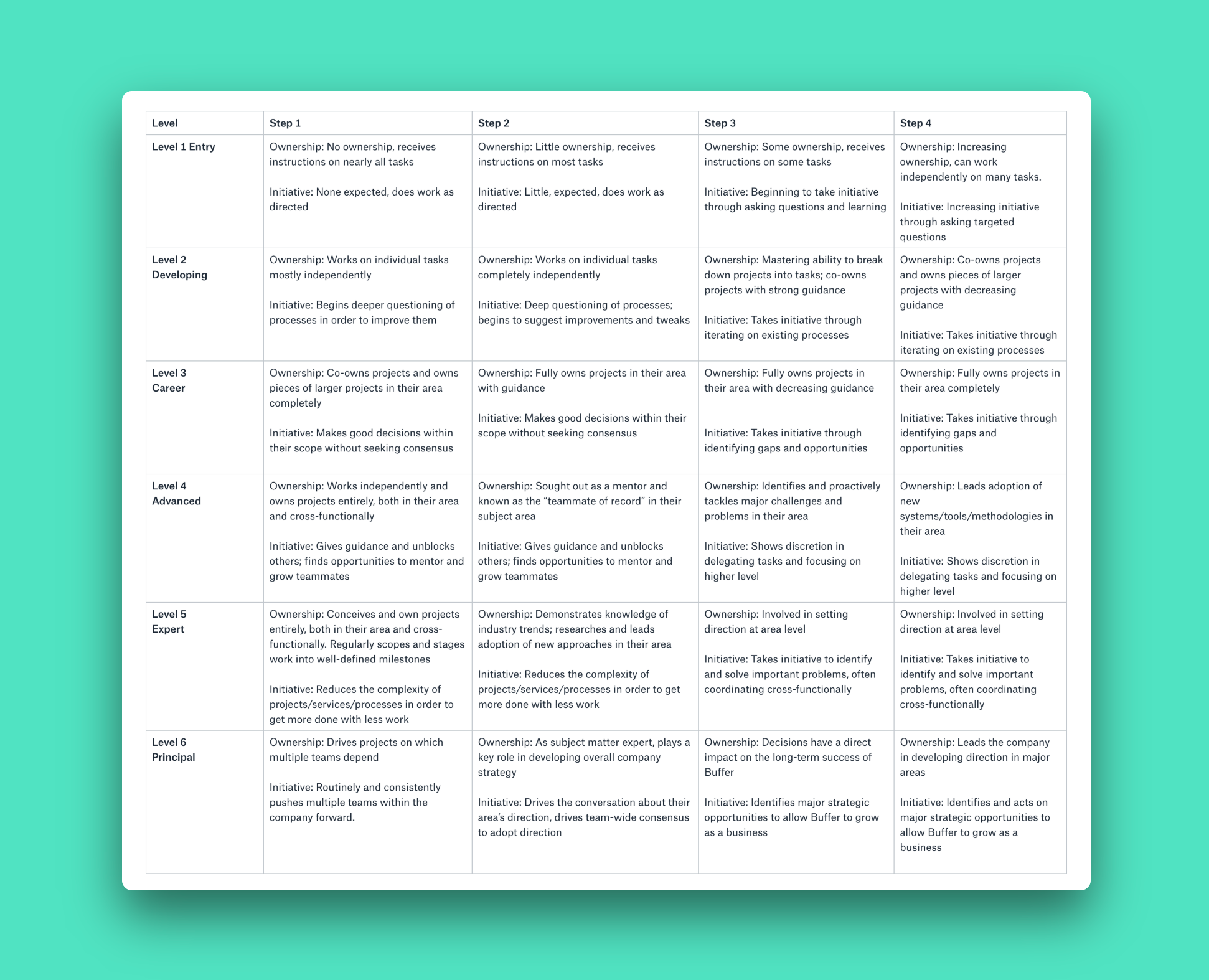
How Individuals Advance At Buffer Without Becoming Managers



