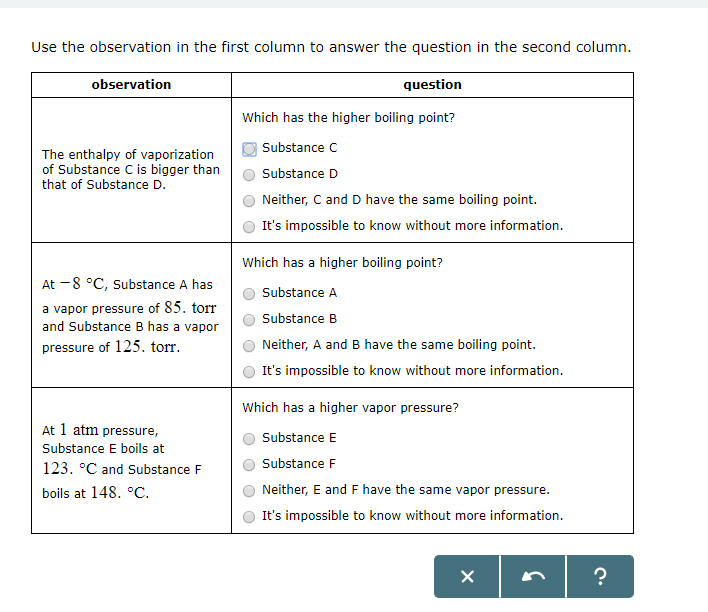
At Pressure Substance C Boils At And Substance D Boils At
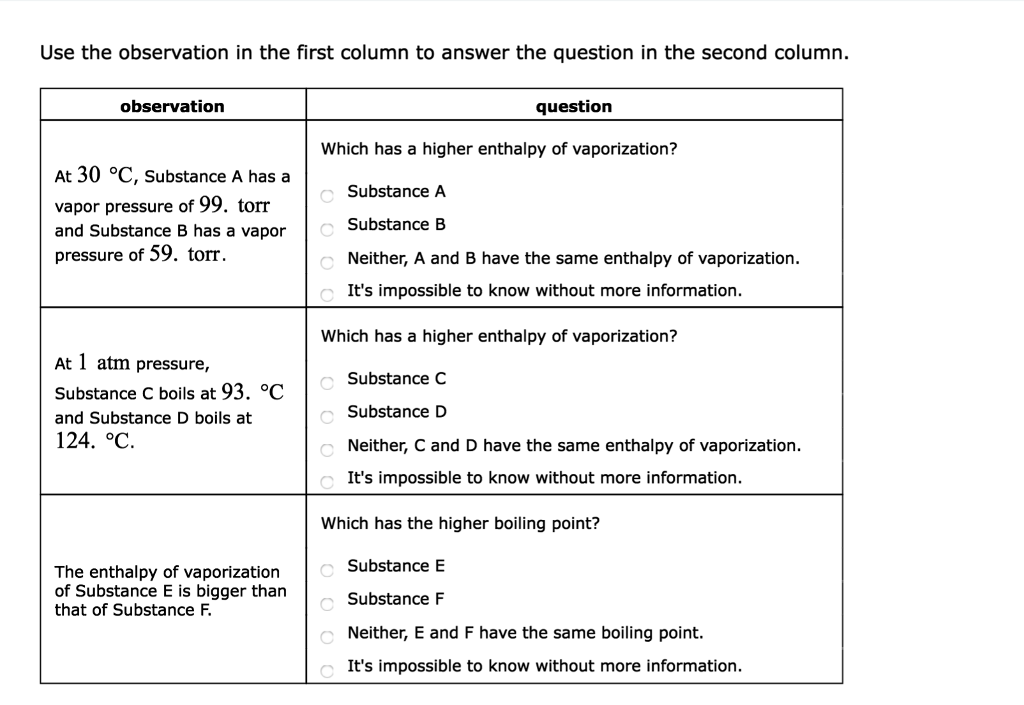
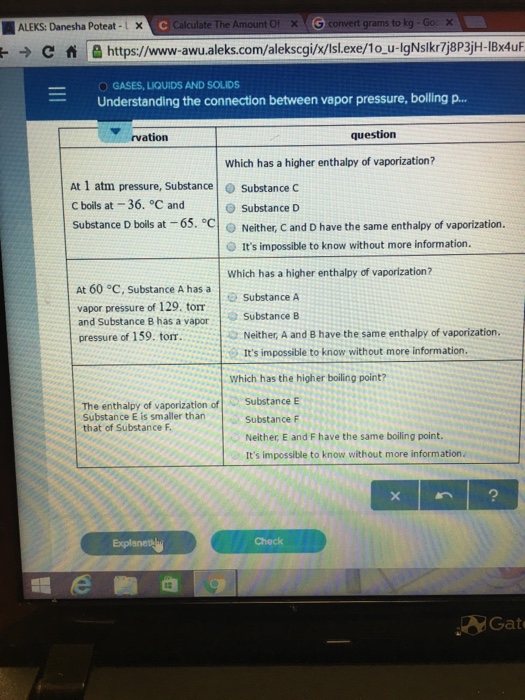
Question Which has a higher enthalpy of vaporization?.

At pressure substance c boils at and substance d boils at. They give the temperature at which the vapor pressure of. A liquid boils when its. LiF - As noted, this is the highest boiling point substance at 1676°.
The approximate normal boiling point of this substance is:. Substance C Substance D Neither, C and D have the same enthalpy of vaporization. A substance has a standard boiling point of 78.90 °C.
A plot of the natural log of the vapor pressure versus the inverse of the temperature (in Kelvin) produces a straight line with a slope of -42.5 K. Air pressure above the substance. This means that the vapor pressure of water is 1 atm or 760 mmHg at 100 оC.
The vapor pressure of most liquids has a fairly predictable temperature-dependence, so from one boiling point measurement it is possible to give a good estimation of the boiling point at other pressures (or boiling pressure at other temperatures). What is the enthalpy of vaporization in kJ mol−1?. Atmospheric pressure in inches of mercury (" Hg) decreases by _____ inch(s) per 1000 feet increase in elevation.
So,if the pressure of a substance is increased during a boiling process then temperature is also getting change. At 59 °C, Substance A has a Substance A vapor pressure of 85. E) Raising the pressure from point E to point C causes the substance to condense.
A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. At a pressure of 1.5 atm, the. Dimethyl ether boils at −23.7°C.
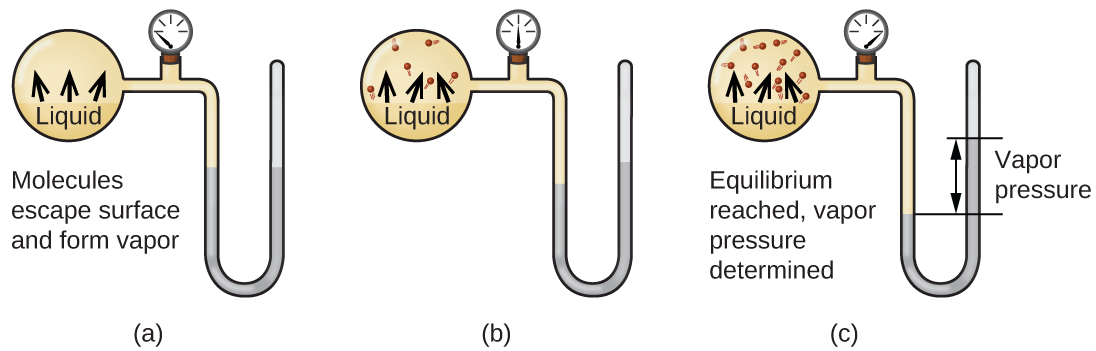
The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure, thus facilitating transition of the material between gaseous and liquid phases. The vapour pressure is the pressure exerted when the molecules leave the surface at the same rate as they return. What is the enthalpy of vaporization of the substance?.
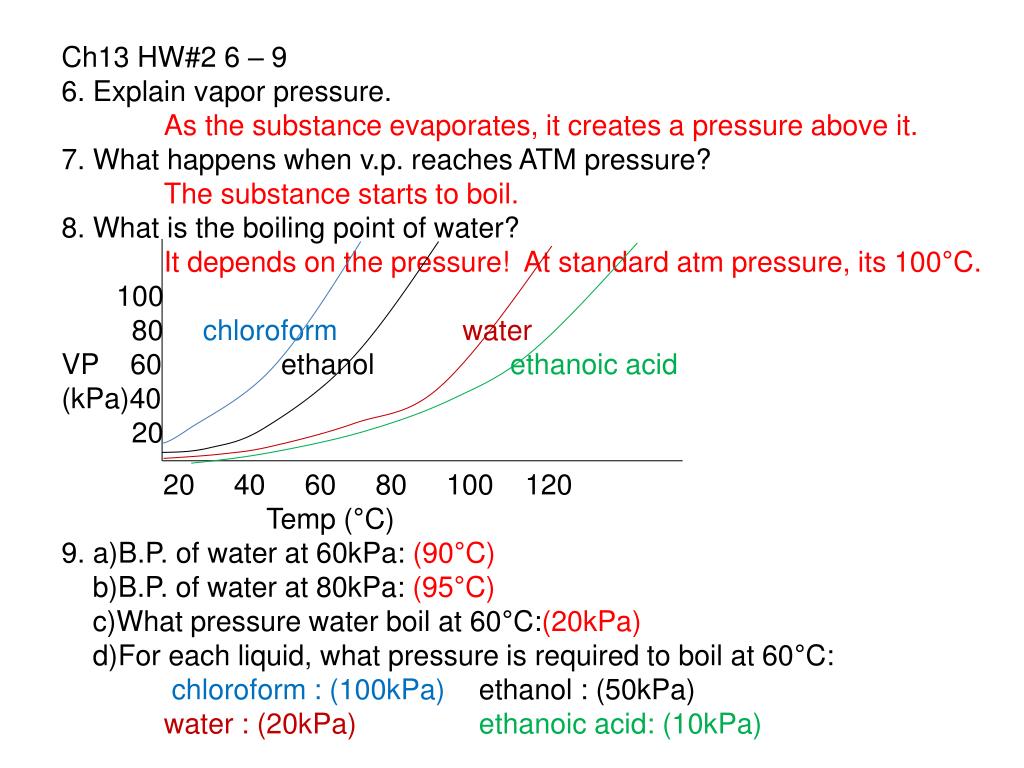
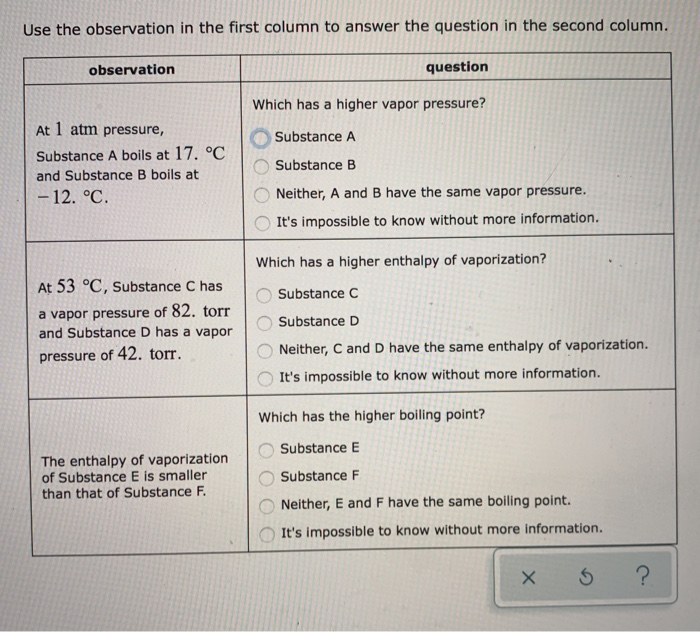
Torr and Substance D has a vapor pressure of 186. The boiling point decreases as the vapour pressure increases. From ideal gas equation we have, Where n is the Number of moles and R is the gas.
Both of these d. (image on other side) A) 430K B) 180 K C) 190 K D) 300 K. Substance C Substance D Neither, C and D have the same enthalpy of vaporization.
Freezing point is greater than melting point for a pure substance c. Which has a higher boiling point?. You also need the melting point (m.p.).
What will the vapor pressure be at 25.00 °C?. C) D) E) answer 6) The vapor pressure of any substance at its normal boiling point is 6) A) 1 torr B) equal to the vapor pressure of water C) equal to atmospheric pressure D) 1 Pa E) 1 atm 7) Some things take longer to cook at high altitudes than at low altitudes because _____. C) the temperature at which water boils.
Observation At 25 degree C, Substance C has a vapor pressure of 136. If the temperature is raised from 50 K to 400 K at a pressure of I atm, the substance boils at approximately 185 K. Increasing the external pressure requires a greater matching vapour pressure that is only provided at a higher vapour (and liquid) temperature as the molecules mean speed is increased.
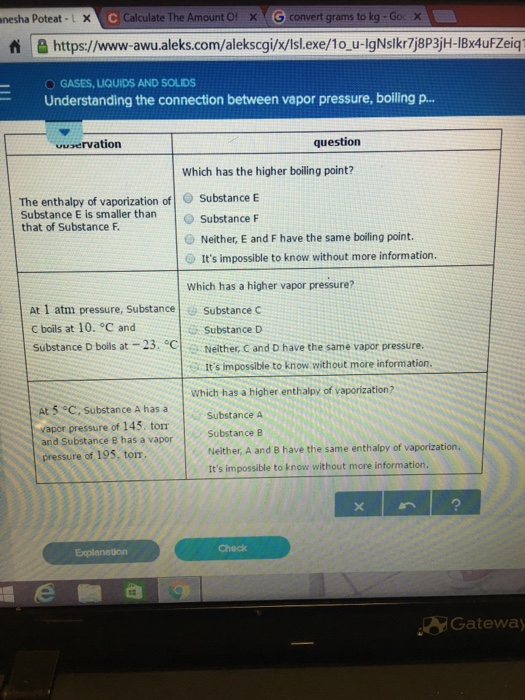
Substance E Substance F Neither, C and D have the same boiling point. Arrange these substances in order of INCREASING boiling point:. The change of phase of a substance from liquid to gas is known as evaporation.
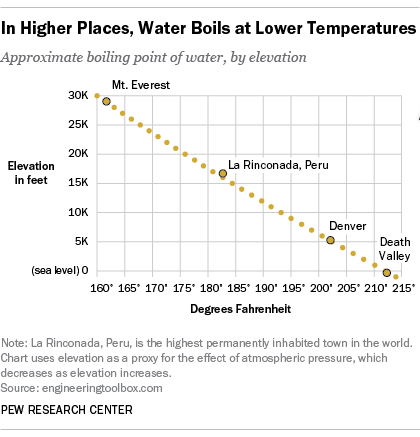
At higher altitudes the air pressure is lower. The liquid phase of this substance cannot exist under conditions of 2 atm at any temperature. Freezing point is less than melting point for a pure substance b.
Degree C Which has a higher vapor pressure?. Solutions and Vapor Pressure. If the pressure of a substance is increased during a boiling process, will the temperature also increase or will it remain constant?.
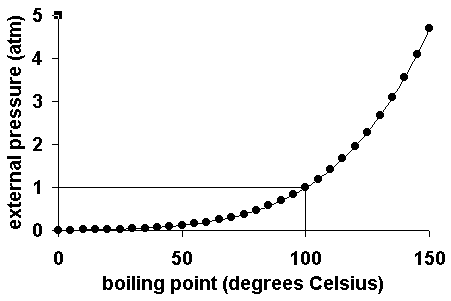
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes how the pressure of a gas tends to increase as the volume of the container decreases. The normal boiling point is specified when the ambient pressure is 1 ⋅atm. At every point along this line, the liquid boils to form a gas and the gas condenses to form a liquid at the same rate.
This pressure decreases with increasing elevation and hence the boiling point of a liquid also decreases. Decreasing the air pressure above the water c. For example, the triple point of mercury occurs at a temperature of −38.440 °C (−37.
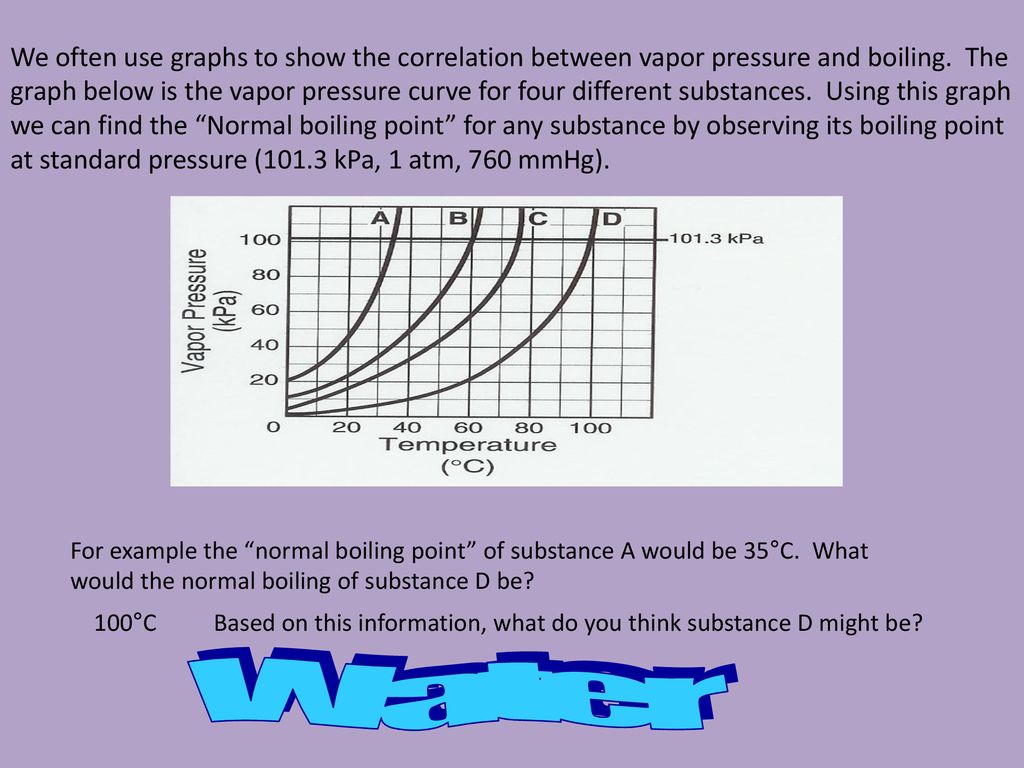
Melting begins _____ c. Learn how to get rid of a boil and what you can do at home and with your doctor to treat and prevent future boils. The normal boiling point for A is about 60˚ d.
2,47 x 1023 kJ/mol C. It contains all of the combinations of temperature and pressure at which the liquid boils. Determine the vapor pressure (in mm Hg) of a substance at 29°C, whose normal boiling point is 76°C and has a ΔHvap of 38.7 kJ/mol.?.
Torr It's impossible to know without more information. The inverse as you decrease the pressure the temperature required to boil is also reduced. 1 "When water boils at 212 F boils, it is only absorbing latent heat.".
The boiling point of a liquid varies depending upon the surrounding environmental pressure. Torr and Substance B has a vapor Substance B Neither, A and B have the same boiling point. Freezing begins _____ d.
Water boils in a pressure cooker c. D) the pressure at which a liquid boils at 273.15 K. C) Point C corresponds to the liquid phase of the substance.
When you have a liquid (in 1 atmosphere of air pressure) we define its boiling point when its vapor pressure is equal to atmospheric pressure. Is above room temperature, and the m.p. Does the substance give off or take in heat as it goes from D to C?.
The boiling point of a pure liquid _____ as external pressure decreases. D) temperature is greater than room temperature. Time-lapse entry of liquid into the capillary tube.
Both freezing point and melting point are the same for a pure substance. We expect water to boil at 100 o C when we cook, but in Denver, Colorado, which is a mile high and has a lower atmospheric pressure than at sea level, water boils at a lower temperature. Internal volume of the substance B.
It's impossible to know without more information. It's impossible to know without more information. Ethanol boils at 78.4°C.
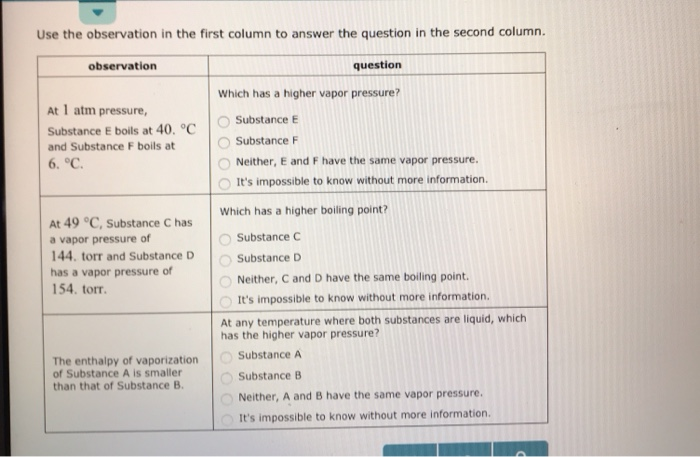
It's impossible to know without more information. At 17 degree C, substance C has a vapor pressure of 125. A pure substance has well-defined physical properties such as freezing and boiling points.
To achieve a vapor pressure of 0.6 atm, substance D must be heated to about 60˚C 25. Substance E Substance F Neither, A and B have the same boiling point. Because the boiling point of a liquid rises with pressure, the contents of the pressurized vessel can remain liquid so long as the vessel is intact.
(b) the temperature above which the substance cannot exist as a liquid regardless of the pressure. The vapor pressure would have to increase to equal the higher atmospheric pressure for the substance to boil. (c) HBr (d) HI (e) H 2 SO 4.
The boiling point is the easier concept to think about. 1)freeze 2)boil 3)melt 4)condense 8.When the vapor pressure of a liquid is equal to the atmospheric pressure, the liquid will 9.The graph below shows the relationship between vapor pressure and temperature for substance X. Substance C Substance D neither, C and D have the same boiling point.
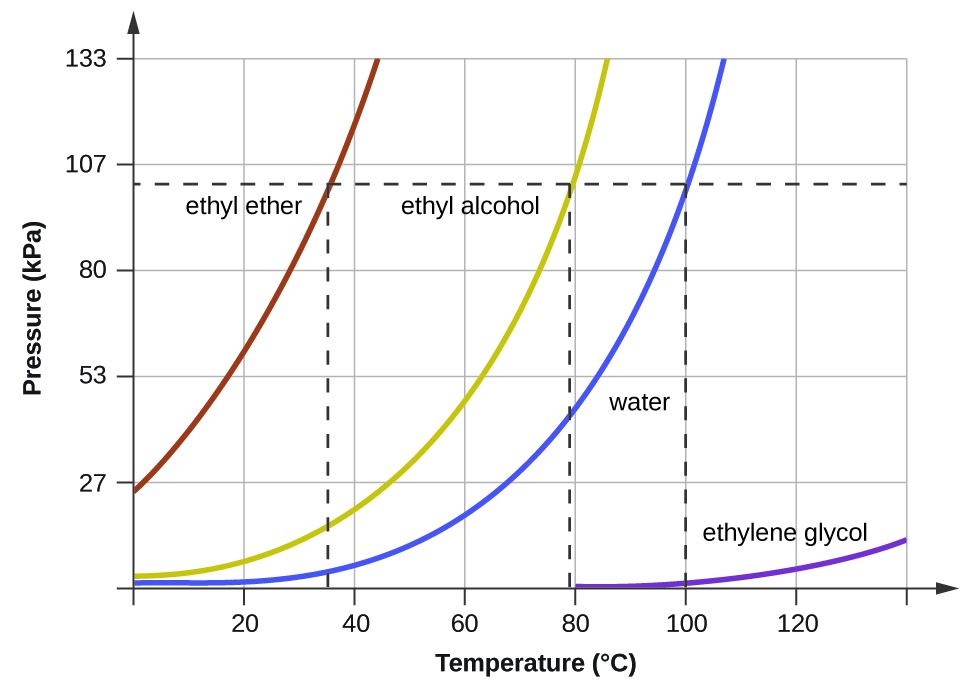
Explain why the boiling point of the ether is so much lower than the boiling point of ethanol. The same substance has a vapor pressure at −21.09 °C of 0.101 bar. At 5 degree C, Substance A has a vapor pressure of 145, torr and Substance 8 has a vapor pressure of 195 torr.
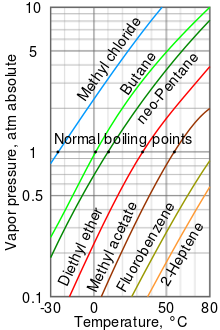
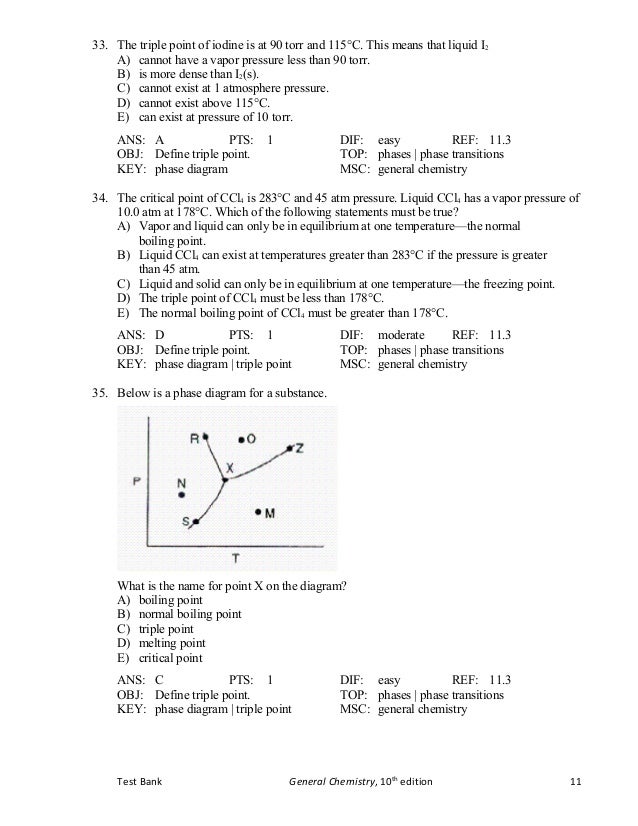
For the vapor pressure/temperature diagram shown, approximate the normal boiling points for:. ΔH vap = 24. Of course, if we reduce the ambient pressure, the boiling point will be substantially reduced, and this is an example of the principle of vacuum distillation, where an otherwise involatile liquid is distilled under high vacuum.
A modern statement of Boyle's law is:. A boiling liquid expanding vapor explosion (BLEVE, / ˈ b l ɛ v iː / BLEV-ee) is an explosion caused by the rupture of a vessel containing a pressurized liquid that has reached temperatures above its boiling point. In contrast, many properties of a solution depend on the concentration of solute.Properties of solutions that depend on the concentration (but not on the identity) of solute are called colligative (collective) properties.You will learn how the concentration of a solute.
Is reached when v.p. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. 7 psia and 760 mm Hg - (o F, o C)-3.
It's Impossible to know without more information. C) temperature is equal to 273 K (standard temperature). (c) the temperature at which the gas molecules have more kinetic energy than the molecules in the liquid.
Which has the higher boling point?. > Vapour Pressure Some of the molecules at the surface of a liquid have enough kinetic energy to escape into the atmosphere. Boiling begins _____ b.
Is below room temperature, it’s a liquid. 7) A) natural gas flames don't burn as hot at high altitudes. Applying a great deal of heat.
Weight of the substance C. Their structural formulas are, respectively, CH 3CH 2OH and CH 3OCH 3. For example, for water, the boiling point is 100ºC at a pressure of 1 atm.
E) the sum of the enthalpies of vaporization and fusion at 298 K. Which has a higher enthalpy of vaporization?. The boiling point is defined as the temperature at which the vapour pressure of the liquid is equal to the ambient pressure, and bubbles of vapour form directly in the liquid.
Determine the normal boiling point of a substance whose vapor pressure is 55. The normal boiling point of a liquid is (a) the temperature at which the vapor pressure equals 760 torr. Foreign ions introduced to water a.
The solid line between points B and C is identical to the plot of temperature dependence of the vapor pressure of the liquid. Boils are painful, red bumps on the skin that are caused by bacteria. All boiling points below are normal/atmospheric boiling points:.
The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the. D) At a higher temperature and pressure than point D, the substance exists as a supercritical fluid. B) vapor pressure is equal to, or greater than, the external pressure pushing on it.
It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet. Increasing the air pressure on the water b. When the atmospheric pressure is equal to the vapor pressure of the liquid, boiling will begin.
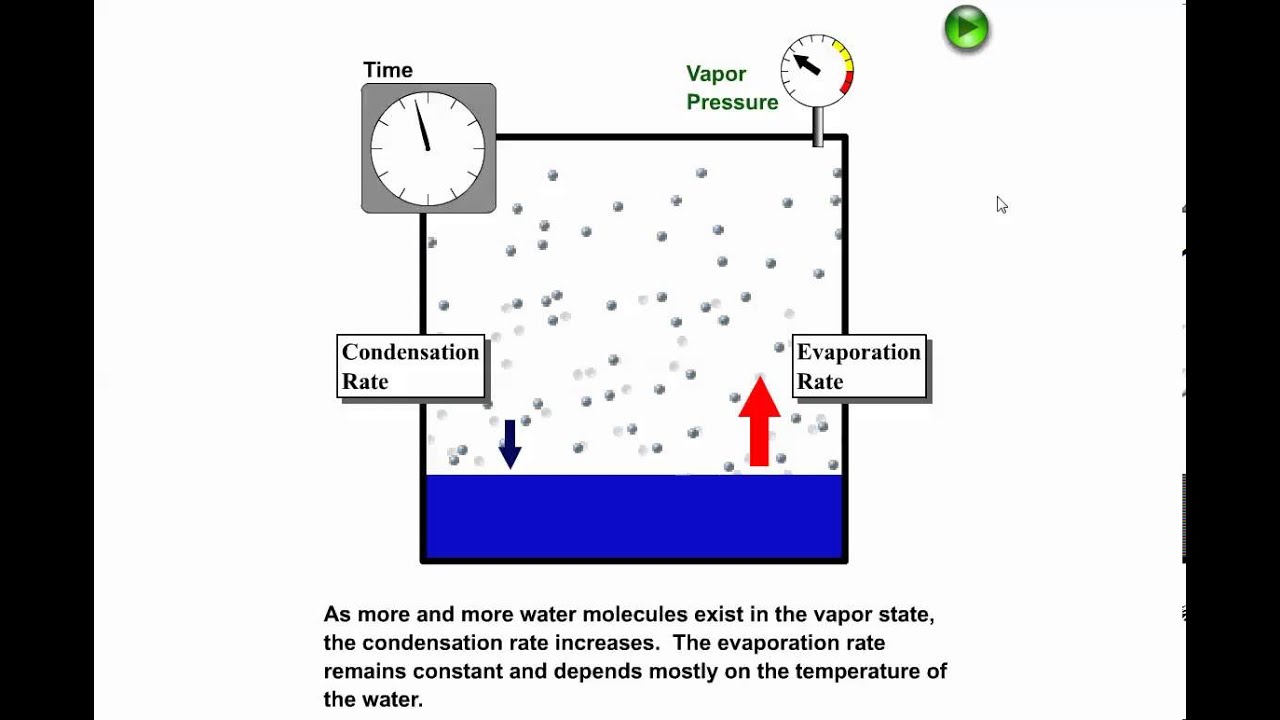
The vapor pressure of a substance is measured over a range of temperatures. A liquid boils when the vapor pressure above a liquid equals the atmospheric pressure. The vapor pressure of a substance is related to temperature, so increasing the temperature increases the vapor pressure till it =1 atmosphere and starts boiling.
Determine the vapor pressure (in mm Hg) of a substance at 29°C, whose normal boiling point is 76°C and has a Hvapof 38.7 kJ/mol. The vapor pressure of a substance depends on the temperature. Using the following phase diagram of a certain substance, in what phase is the substance at 50°C and 1 atm.
These molecules exert a pressure on the walls of a closed container. Water could be made to boil at 105°C instead of 100°C by _____. The triple point occurs at approximately 165 K.
Evaporation can occur at any temperature, but takes place only at the surface of the liquid. Xe, H 2, H 2 O, LiCl, H 2 S. Since it exists as a gas at room temperature, it obviously has a low boiling point, the lowest of the group, which is -1°C.
Boiling Point, 75-79 °C/12 mmHg. Solution for If the pressure of a substance is increased during a boiling process, will the temperature also increase or will it remain constant?. A) vapor pressure is exactly 1 atmosphere.
The onset of boiling occurs when the vapour pressure inside the bubble equals the external pressure that acts to collapse it. _____ Figure 2 E Liquid Gas Solid Liquid Solid Liquid Gas A B80 C D F G Energy e 0 40 60 40 60 100 Temperature (°C) CHCl 3 CCl 4 H 2 O Figure 1 101.325 kPa ). To understand it more clearly, recall that the boiling point of water is 100 оC at 1 atm.
At 1 atm pressure, Substance C boils at -36 degree C and Substance D boils at -65 degree C Which has a higher enthalpy of vaporization?. B) the temperature at which the vapor pressure of a substance equals 1 atm. The atmospheric pressure at sea level is equal to 101,325 Pa (pascal).
296 g = 0. Torr Which has a higher boiling point?. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. Degree C and Substance D boils at - 23. A) the pressure of a gas when its temperature reaches 373.15 K.
At 1 atm pressure, Substance C boils at 10. Is above room temperature, it’s a. If the boiling point (b.p.) is below room temperature, it’s a gas.
Torr and Substance D has a vapor pressure of 115.
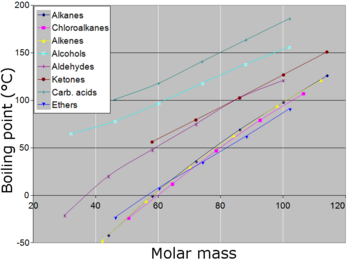
Boiling Point Wikipedia

Solved Which Has A Higher Enthalpy Of Vaporization At 1 Chegg Com
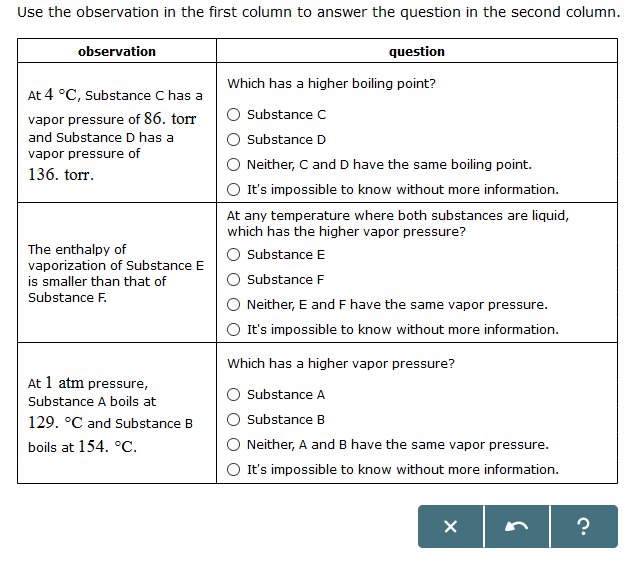
Solved Use The Observation In The First Column To Answer Chegg Com
At Pressure Substance C Boils At And Substance D Boils At のギャラリー
Loganchemistry Weebly Com Uploads 2 4 7 4 Ch 13 Review Answers Pdf

Effect Of Pressure On The Boiling Point Of Water Youtube

Chemistry 1 Using The Clausius Clapeyron Equation To Solve For Vapor Pressure Youtube
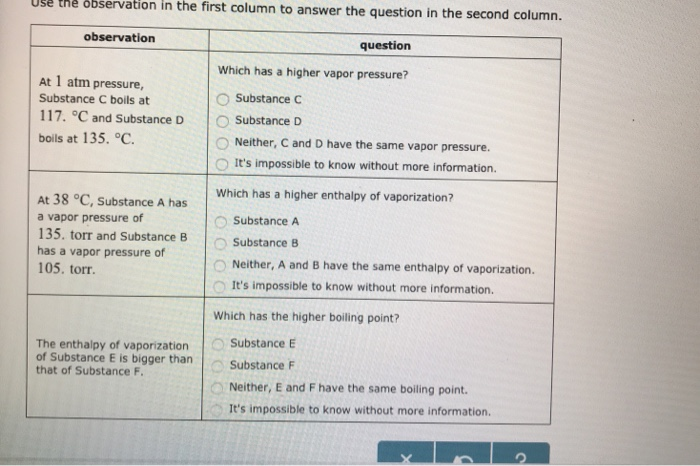
Solved Use The Observation In The First Column To Answer Chegg Com

Boiling Point Elevation And Freezing Point Depression Video Khan Academy

Key Chapter 11 Practice Test
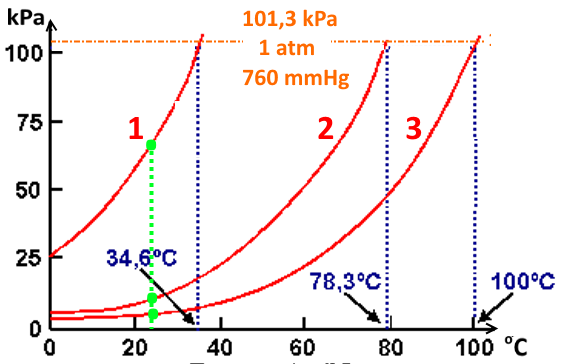
Boiling Of A Liquid Substance
Ppt Chapter 13 States Of Matter Powerpoint Presentation Free Download Id
Http Www2 Chem Uic Edu Tak Chem Solutions set 10 Pdf

Answered The Phase Diagram Of A Substance Is Bartleby

Physical Chemistry Solutions D Elevation In Boiling Point 39 The Vapour Pressure Of Benzene At A Certain Temperature Is 640 Mm Kunduz

Changes Of State Ppt Download

An Aqueous Solution Of A Non Volatile And Non Electrolytic Substance Boils At 100 5 C Calculate The Osmotic Pressure Of This Solution At 27 C Ko For Water Per 1000 G 0 50

Melting Point Freezing Point Boiling Point

Chapter 2a Pure Substances Phase Change Properties Updated 9 09

B I I Ii C I Ii M D I I Ii Q 62 To 64

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb

A Water Freezes At Standard Pressure B Water Is At Its Triple Point C

Solved Use The Observation In The First Column To Answer Chegg Com
Solved Use The Observation In The First Column To Answer The Question In The Second Column Which Has A Higher Vapor Pressure At 1 Atm Pressure S Course Hero

What Are The Freezing Melting And Boiling Points Of Solids Liquids And Gases Owlcation Education

Oneclass 1 Define Triple Point A The Temperature That Is Unique For A Substance B The Temperatu

Boiling Point Accessscience From Mcgraw Hill Education
Changes Of State Ppt Download
Solved Use The Observation In The First Column To Answer Chegg Com

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb

Boiling Point Definition Examples Temperature Facts Britannica

A Solution Containing 3 3 G Of A Substance In 125 G Of Benzene B Pt 80 Oc Boils At 80 66 Oc If Kb For Benzene Is 3 28 K Kg Mol 1 The Molecular

Boiling Chemistry Libretexts

Answered When Two Chemicals Have The Same Bartleby

Question 3 I A Design An Experiment To Show That Ammonium Chloride Undergoes S A Diagram Give Two Science Matter In Our Surroundings Meritnation Com
Phase Changes Boundless Chemistry

Answered If A Substance Has Stronger Bartleby
Solved Use The Observation In The First Column To Answer Chegg Com
2

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb

Chemistry 1 Using The Clausius Clapeyron Equation To Solve For Vapor Pressure Youtube
Boiling Point Wikipedia
Properties Of Liquids
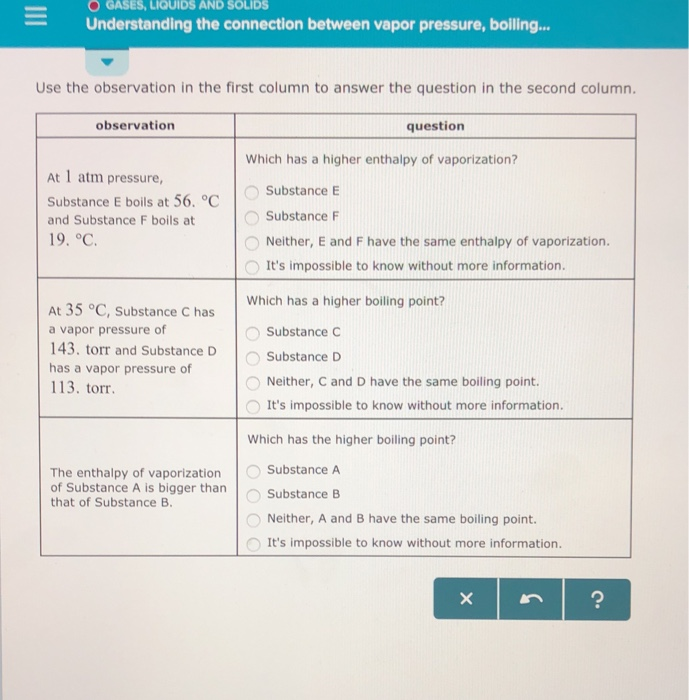
Solved Gases Liquids And Solids Understanding The Connec Chegg Com
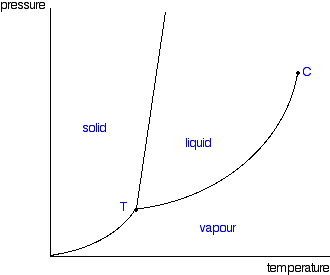
Phase Diagrams Of Pure Substances

Distillation

Phase Diagrams

Vapor Pressure Curves Ck 12 Foundation
/glass-saucepan-on-a-gas-burner-with-boiling-water-dor961844-5a036e3089eacc0037b4abb1.jpg)
Boiling Definition In Chemistry
Www Plps K12 Org Site Handlers Filedownload Ashx Moduleinstanceid 24 Dataid 3863 Filename Practice test chapter 10 key Pdf

An Aqueous Solution Of A Non Volatile And Non Electrolytic Substance Boils At 100 5 C Calculate The Osmotic Pressure Of This Solution At 27 C Ko For Water Per 1000 G 0 50

Boiling Chemistry Libretexts
10 3 Phase Transitions Chemistry Libretexts

Boiling Point Elevation Wikipedia

Which Of The Following Solutions Will Have Highest Boiling Point
Properties Of Liquids

Heating Curve For Water Introduction To Chemistry

Melting Point Wikipedia
Q Tbn And9gcslxxohf6s Byidgpakdcgmarcsqcauhrjvta Usqp Cau

Ppt Chapter 13 States Of Matter Powerpoint Presentation Free Download Id

The Wizard Test Maker Revsworld

Boiling Point Wikipedia
Boiling

Use The Observation In The First Column To Answer The Question In The Second Column Observation Homeworklib

Attractions And Boiling

11 5 Vapor Pressure Chemistry Libretexts
Www Lamar Edu Arts Sciences Files Documents Chemistry Biochemistry Dorris 1412exam2 Pdf
Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb

Solved Use The Observation In The First Column To Answer Chegg Com

15 2d Understanding The Connection Between Vapor Pressure Boiling Point And Enthalpy Of Vaporizati Youtube
Usflearn Instructure Com Courses 9868 Files Download Wrap 1

For The First Three Questions Use The Key Below

Mcq Questions Latent Heat Melting Point
Solved Use The Observation In The First Column To Answer Chegg Com
Characteristics Of The Solid Liquid And Gaseous States
Solved At 1 Atm Pressure Substance C Boils At 36 Degree Chegg Com
Does Water S Boiling Point Change With Altitude Americans Aren T Sure Pew Research Center
Solved Use The Observation In The First Column To Answer Chegg Com
Solved The Enthalpy Of Vaporization Of Substance E Is Sma Chegg Com
Define Melting Point And Boiling Point What Is The Difference Quora

Vapor Pressure Video States Of Matter Khan Academy

Distillation

The Boiling Point Of A Substance Is Affected By A The Mass Of The Substance B The Substance S Brainly Com

Aleks Calculating Vapor Pressure From Boiling Point And Enthalpy Of Vaporization Youtube
10 3 Phase Transitions Chemistry Libretexts
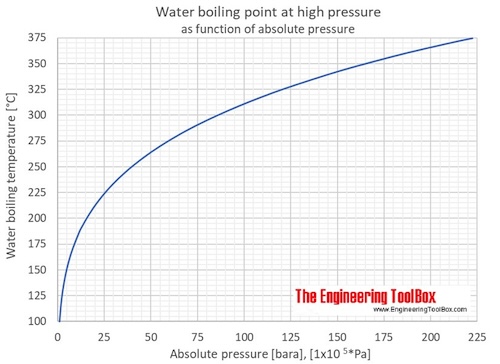
Water Boiling Points At Higher Pressure
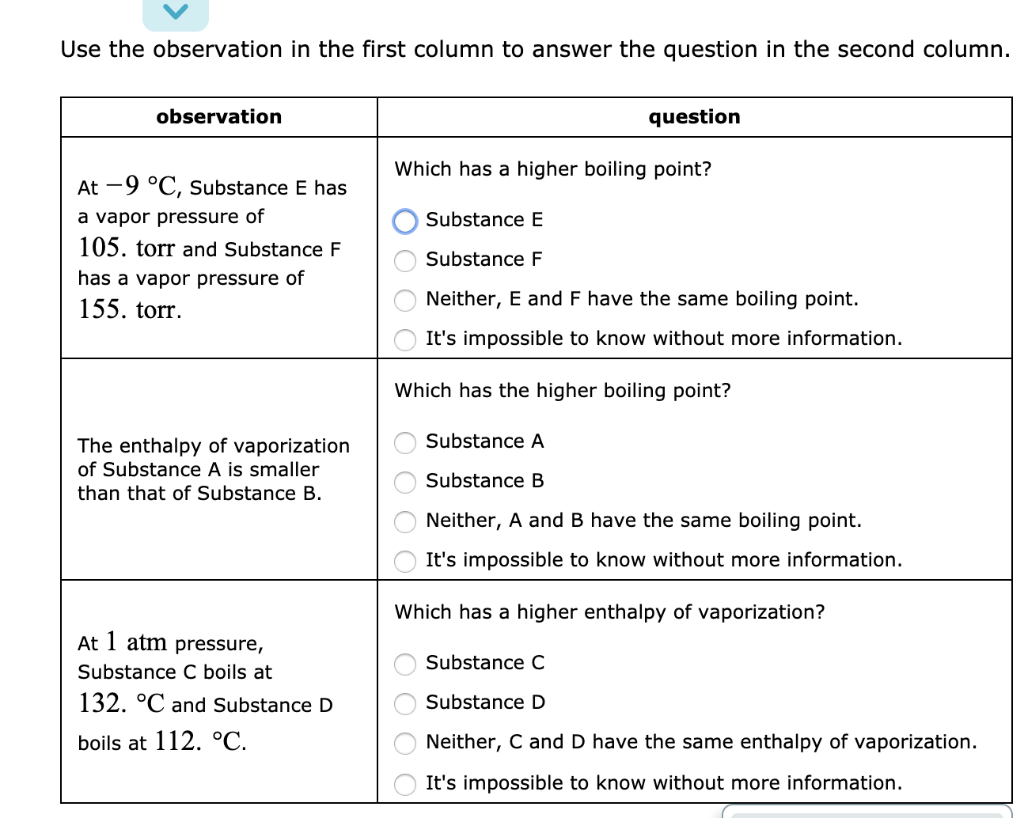
Solved Use The Observation In The First Column To Answer Chegg Com

Oneclass 1 What Type Or Types Of Intermolecular Forces Are Present Between Linear Molecules Of H Ce

Gases Liquids And Solids Understanding The Connection Between Use The Observation In The First Column Homeworklib
Vapor Pressure Wikipedia
Http Cdochemistrychristman Pbworks Com W File Fetch Ap
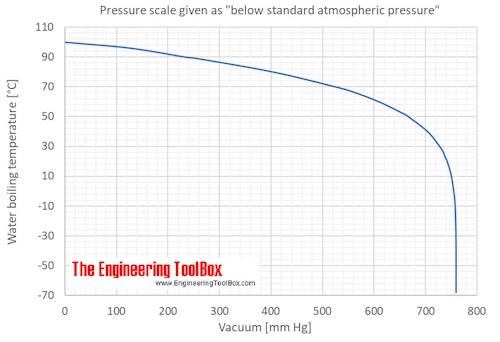
Water Boiling Points At Vacuum Pressure
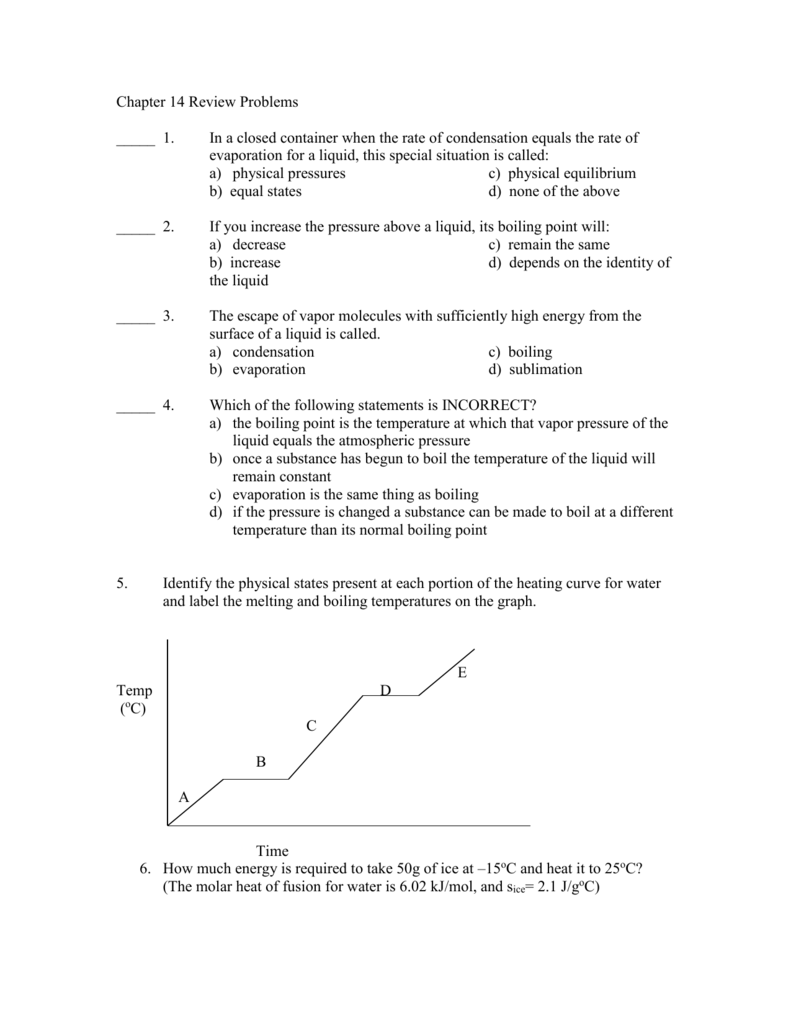
Chapter 14 Review Problems

Oneclass Heat Of Vaporization Substance Would You Expect To Have The Highest 3 Pts A F2 B C12 C
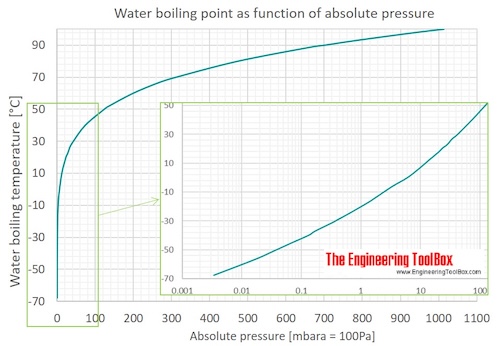
Water Boiling Points At Vacuum Pressure

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb
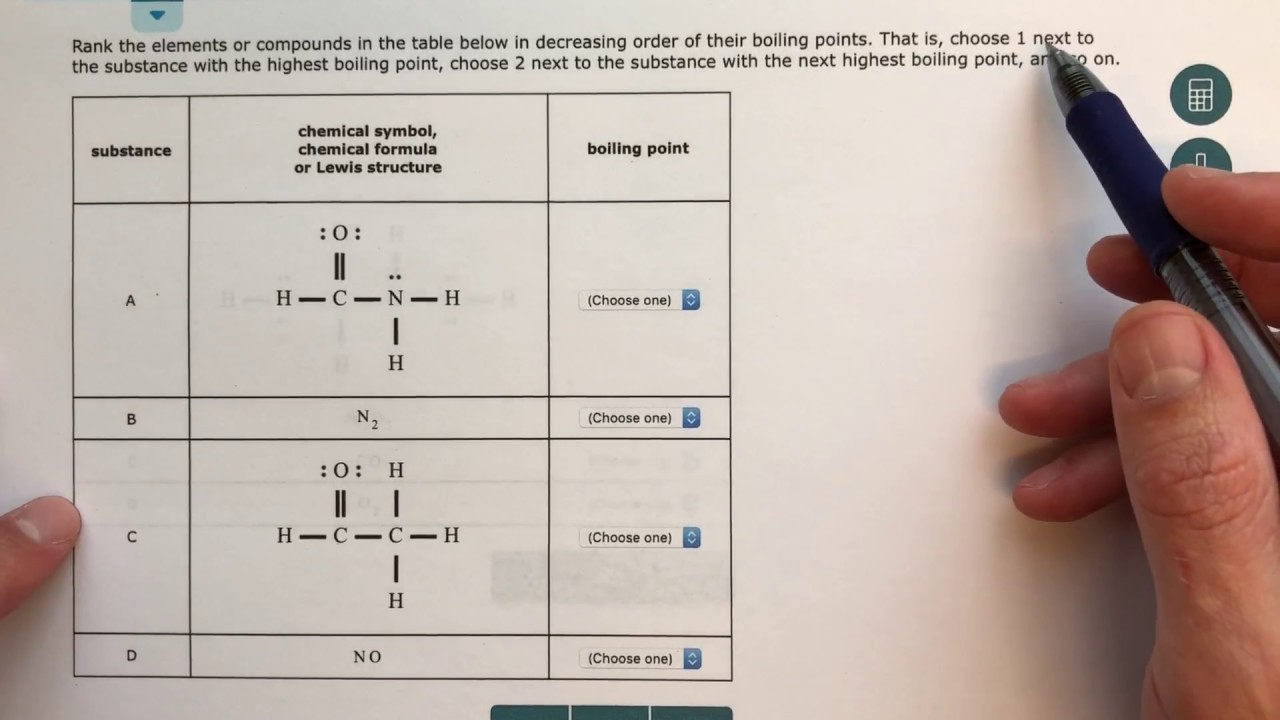
Aleks Predicting The Relative Boiling Points Of Pure Substances Youtube
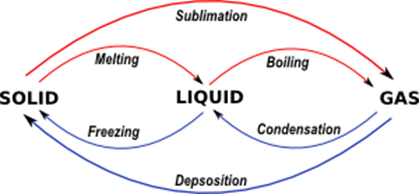
Change Of State Of A Matter Melting Fusing Evaporation Condensation
Characteristics Of The Solid Liquid And Gaseous States
A Vapor Pressure Lowering

Solved Use The Observation In The First Column To Answer Chegg Com



